Renal fibrosis: collagen composition and assembly regulates epithelial-mesenchymal transdifferentiation
- PMID: 11583959
- PMCID: PMC1850511
- DOI: 10.1016/S0002-9440(10)62518-7
Renal fibrosis: collagen composition and assembly regulates epithelial-mesenchymal transdifferentiation
Abstract
Type IV collagen is a major component of basement membranes and it provides structural and functional support to various cell types. Type IV collagen exists in a highly complex suprastructure form and recent studies implicate that protomer (the trimeric building unit of type IV collagen) assembly is mediated by the NC1 domain present in the C-terminus of each collagen alpha-chain polypeptide. Here we show that type IV collagen contributes to the maintenance of the epithelial phenotype of proximal tubular epithelial cells, whereas type I collagen promotes epithelial-to-mesenchymal transdifferentiation (EMT). In addition, the recombinant human alpha1NC1 domain inhibits assembly of type IV collagen NC1 hexamers and potentially disrupts the deposition of type IV collagen, facilitating EMT in vitro. Inhibition of type IV collagen assembly by the alpha1NC1 domain up-regulates the production of transforming growth factor-beta1 in proximal tubular epithelial cells, an inducer of EMT. These results strongly suggest that basement membrane architecture is pivotal for the maintenance of epithelial phenotype and that changes in basement membrane architecture potentially lead to up-regulation of transforming growth factor-beta1, which contributes to EMT during renal fibrosis.
Figures




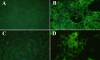
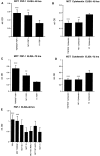
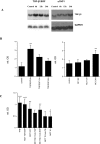
Comment in
-
Transforming growth factor-beta, basement membrane, and epithelial-mesenchymal transdifferentiation: implications for fibrosis in kidney disease.Am J Pathol. 2001 Oct;159(4):1187-92. doi: 10.1016/s0002-9440(10)62503-5. Am J Pathol. 2001. PMID: 11583944 Free PMC article. Review. No abstract available.
References
-
- Yurchenco PD, O’Rear JJ: Basal lamina assembly. Curr Opin Cell Biol 1994, 6:674-681 - PubMed
-
- Hudson BG, Reeders ST, Tryggvason K: Type IV collagen: structure, gene organization, and role in human diseases. Molecular basis of Goodpasture and Alport syndromes and diffuse leiomyomatosis. J Biol Chem 1993, 268:26033-26036 - PubMed
-
- Timpl R, Oberbaumer I, von der Mark H, Bode W, Wick G, Weber S, Engel J: Structure and biology of the globular domain of basement membrane type IV collagen. Ann NY Acad Sci 1985, 460:58-72 - PubMed
-
- Yurchenco PD: Assembly of basement membranes. Ann NY Acad Sci 1990, 580:195-213 - PubMed
-
- Kalluri R, Cosgrove D: Assembly of type IV collagen. Insights from alpha3(IV) collagen-deficient mice. J Biol Chem 2000, 275:12719-12724 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

