Suppression by apoptotic cells defines tumor necrosis factor-mediated induction of glomerular mesangial cell apoptosis by activated macrophages
- PMID: 11583967
- PMCID: PMC1850510
- DOI: 10.1016/S0002-9440(10)62526-6
Suppression by apoptotic cells defines tumor necrosis factor-mediated induction of glomerular mesangial cell apoptosis by activated macrophages
Abstract
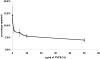
Activated macrophages (M(phi)) isolated from inflamed glomeruli or generated by interferon-gamma and lipopolysaccharide treatment in vitro induce glomerular mesangial cell apoptosis by hitherto incompletely understood mechanisms. In this report we demonstrate that nitric oxide-independent killing of co-cultured mesangial cells by interferon-gamma/lipopolysaccharide-activated M(phi) is suppressed by binding/ingestion of apoptotic cells and is mediated by tumor necrosis factor (TNF). Thus, soluble TNF receptor-1 significantly inhibited induction of mesangial cell apoptosis by 1) rodent M(phi) in the presence of nitric oxide synthase inhibitors or 2) human M(phi), both situations in which nitric oxide release was minimal. Furthermore, murine TNF knockout M(phi) were completely unable to induce mesangial cell apoptosis in the presence of nitric oxide synthase inhibitors. We conclude that TNF-restricted M(phi)-directed apoptosis of glomerular mesangial cells can be down-regulated by M(phi) binding/ingestion of apoptotic cells, suggesting a new mechanism for negative feedback regulation of M(phi) controls on resident cell number at inflamed sites.
Figures




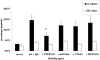

References
-
- Schocklmann HO, Lang S, Sterzel RB: Regulation of mesangial cell proliferation. Kidney Int 1999, 56:1199-1207 - PubMed
-
- Shimizu A, Masuda Y, Kitamura H, Ishizaki M, Sugisaki Y, Yamanaka N: Apoptosis in progressive crescentic glomerulonephritis. Lab Invest 1996, 74:941-951 - PubMed
-
- Duffield JS, Erwig LP, Wei X, Liew FY, Rees AJ, Savill JS: Activated macrophages direct apoptosis and suppress mitosis of mesangial cells. J Immunol 2000, 164:2110-2119 - PubMed
-
- Meszaros AJ, Reichner JS, Albina JE: Macrophage-induced neutrophil apoptosis. J Immunol 2000, 165:435-441 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

