Mitochondrial alterations caused by defective peroxisomal biogenesis in a mouse model for Zellweger syndrome (PEX5 knockout mouse)
- PMID: 11583975
- PMCID: PMC1850512
- DOI: 10.1016/S0002-9440(10)62534-5
Mitochondrial alterations caused by defective peroxisomal biogenesis in a mouse model for Zellweger syndrome (PEX5 knockout mouse)
Abstract
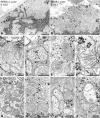
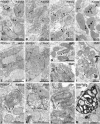
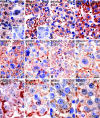
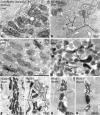
Zellweger syndrome (cerebro-hepato-renal syndrome) is the most severe form of the peroxisomal biogenesis disorders leading to early death of the affected children. To study the pathogenetic mechanisms causing organ dysfunctions in Zellweger syndrome, we have recently developed a knockout-mouse model by disrupting the PEX5 gene, encoding the targeting receptor for most peroxisomal matrix proteins (M Baes, P Gressens, E Baumgart, P Carmeliet, M Casteels, M Fransen, P Evrard, D Fahimi, PE Declercq, D Collen, PP van Veldhoven, GP Mannaerts: A mouse model for Zellweger syndrome. Nat Genet 1997, 17:49-57). In this study, we present evidence that the absence of functional peroxisomes, causing a general defect in peroxisomal metabolism, leads to proliferation of pleomorphic mitochondria with severe alterations of the mitochondrial ultrastructure, changes in the expression and activities of mitochondrial respiratory chain complexes, and an increase in the heterogeneity of the mitochondrial compartment in various organs and specific cell types (eg, liver, proximal tubules of the kidney, adrenal cortex, heart, skeletal and smooth muscle cells, neutrophils). The changes of mitochondrial respiratory chain enzymes are accompanied by a marked increase of mitochondrial manganese-superoxide dismutase, as revealed by in situ hybridization and immunocytochemistry, suggesting increased production of reactive oxygen species in altered mitochondria. This increased oxidative stress induced probably by defective peroxisomal antioxidant mechanisms combined with accumulation of lipid intermediates of peroxisomal beta-oxidation system could contribute significantly to the pathogenesis of multiple organ dysfunctions in Zellweger syndrome.
Figures


References
-
- Baes M, Gressens P, Baumgart E, Carmeliet P, Casteels M, Fransen M, Evrard P, Fahimi D, Declercq PE, Collen D, van Veldhoven PP, Mannaerts GP: A mouse model for Zellweger syndrome. Nat Genet 1997, 17:49-57 - PubMed
-
- De Duve C, Baudhuin P: Peroxisomes (microbodies and related particles). Physiol Rev 1966, 46:323-357 - PubMed
-
- Singh I: Mammalian peroxisomes: metabolism of oxygen and reactive oxygen species. Ann NY Acad Sci 1996, 804:612-627 - PubMed
-
- Schrader M, Wodopia R, Fahimi HD: Induction of tubular peroxisomes by UV irradiation and reactive oxygen species in HepG2 cells. J Histochem Cytochem 1999, 47:1141-1148 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

