Dynamin isoform-specific interaction with the shank/ProSAP scaffolding proteins of the postsynaptic density and actin cytoskeleton
- PMID: 11583995
- PMCID: PMC2715172
- DOI: 10.1074/jbc.M104927200
Dynamin isoform-specific interaction with the shank/ProSAP scaffolding proteins of the postsynaptic density and actin cytoskeleton
Abstract
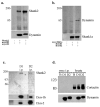
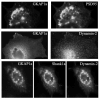
Dynamin is a GTPase involved in endocytosis and other aspects of membrane trafficking. A critical function in the presynaptic compartment attributed to the brain-specific dynamin isoform, dynamin-1, is in synaptic vesicle recycling. We report that dynamin-2 specifically interacts with members of the Shank/ProSAP family of postsynaptic density scaffolding proteins and present evidence that dynamin-2 is specifically associated with the postsynaptic density. These data are consistent with a role for this otherwise broadly distributed form of dynamin in glutamate receptor down-regulation and other aspects of postsynaptic membrane turnover.
Figures



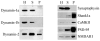
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

