Transforming growth factor beta1 (TGF-beta1) promotes endothelial cell survival during in vitro angiogenesis via an autocrine mechanism implicating TGF-alpha signaling
- PMID: 11585905
- PMCID: PMC99897
- DOI: 10.1128/MCB.21.21.7218-7230.2001
Transforming growth factor beta1 (TGF-beta1) promotes endothelial cell survival during in vitro angiogenesis via an autocrine mechanism implicating TGF-alpha signaling
Abstract
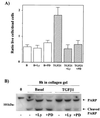
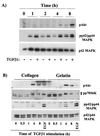
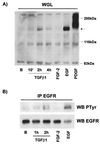
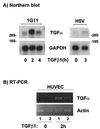
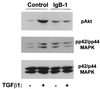
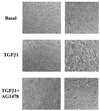
Mouse capillary endothelial cells (1G11 cell line) embedded in type I collagen gels undergo in vitro angiogenesis. Cells rapidly reorganize and form capillary-like structures when stimulated with serum. Transforming growth factor beta1 (TGF-beta1) alone can substitute for serum and induce cell survival and tubular network formation. This TGF-beta1-mediated angiogenic activity depends on phosphatidylinositol 3-kinase (PI3K) and p42/p44 mitogen-activated protein kinase (MAPK) signaling. We showed that specific inhibitors of either pathway (wortmannin, LY-294002, and PD-98059) all suppressed TGF-beta1-induced angiogenesis mainly by compromising cell survival. We established that TGF-beta1 stimulated the expression of TGF-alpha mRNA and protein, the tyrosine phosphorylation of a 170-kDa membrane protein representing the epidermal growth factor (EGF) receptor, and the delayed activation of PI3K/Akt and p42/p44 MAPK. Moreover, we showed that all these TGF-beta1-mediated signaling events, including tubular network formation, were suppressed by incubating TGF-beta1-stimulated endothelial cells with a soluble form of an EGF receptor (ErbB-1) or tyrphostin AG1478, a specific blocker of EGF receptor tyrosine kinase. Finally, addition of TGF-alpha alone poorly stimulated angiogenesis; however, by reducing cell death, it strongly potentiated the action of TGF-beta1. We therefore propose that TGF-beta1 promotes angiogenesis at least in part via the autocrine secretion of TGF-alpha, a cell survival growth factor, activating PI3K/Akt and p42/p44 MAPK.
Figures








Similar articles
-
C-Jun-NH2-terminal kinase mediates expression of connective tissue growth factor induced by transforming growth factor-beta1 in human lung fibroblasts.Am J Respir Cell Mol Biol. 2003 Jun;28(6):754-61. doi: 10.1165/rcmb.4892. Am J Respir Cell Mol Biol. 2003. PMID: 12760970
-
Angiotensin II regulation of TGF-beta in murine mesangial cells involves both PI3 kinase and MAP kinase.Ann Clin Lab Sci. 2004 Summer;34(3):277-86. Ann Clin Lab Sci. 2004. PMID: 15487702
-
Activation of the pro-survival phosphatidylinositol 3-kinase/AKT pathway by transforming growth factor-beta1 in mesenchymal cells is mediated by p38 MAPK-dependent induction of an autocrine growth factor.J Biol Chem. 2004 Jan 9;279(2):1359-67. doi: 10.1074/jbc.M306248200. Epub 2003 Oct 23. J Biol Chem. 2004. PMID: 14576166 Free PMC article.
-
Signaling angiogenesis via p42/p44 MAP kinase cascade.Ann N Y Acad Sci. 2000 May;902:187-200. doi: 10.1111/j.1749-6632.2000.tb06313.x. Ann N Y Acad Sci. 2000. PMID: 10865838 Review.
-
Mechanism of transforming growth factor-beta1 signaling:Kidney Int Suppl. 2000 Sep;77:S53-8. Kidney Int Suppl. 2000. PMID: 10997691 Review.
Cited by
-
Transforming growth factor-beta1 causes pulmonary microvascular endothelial cell apoptosis via ALK5.Am J Physiol Lung Cell Mol Physiol. 2009 May;296(5):L825-38. doi: 10.1152/ajplung.90307.2008. Epub 2009 Mar 6. Am J Physiol Lung Cell Mol Physiol. 2009. PMID: 19270180 Free PMC article.
-
Differential role of PTEN in transforming growth factor β (TGF-β) effects on proliferation and migration in prostate cancer cells.Prostate. 2018 Apr;78(5):377-389. doi: 10.1002/pros.23482. Epub 2018 Jan 16. Prostate. 2018. PMID: 29341212 Free PMC article.
-
Biological study of skin wound treated with Alginate/Carboxymethyl cellulose/chorion membrane, diopside nanoparticles, and Botox A.NPJ Regen Med. 2024 Feb 27;9(1):9. doi: 10.1038/s41536-024-00354-2. NPJ Regen Med. 2024. PMID: 38413625 Free PMC article.
-
Mechanisms of tumor growth and metastasis in head and neck squamous cell carcinoma.Curr Treat Options Oncol. 2007 Jun;8(3):227-38. doi: 10.1007/s11864-007-0032-2. Curr Treat Options Oncol. 2007. PMID: 17712533 Review.
-
Cell type-specific transforming growth factor-β (TGF-β) signaling in the regulation of salivary gland fibrosis and regeneration.J Oral Biol Craniofac Res. 2024 May-Jun;14(3):257-272. doi: 10.1016/j.jobcr.2024.03.005. Epub 2024 Mar 21. J Oral Biol Craniofac Res. 2024. PMID: 38559587 Free PMC article.
References
-
- Alroy I, Yarden Y. The ErbB signaling network in embryogenesis and oncogenesis: signal diversification through combinatorial ligand-receptor interactions. FEBS Lett. 1997;410:83–86. - PubMed
-
- Baird A, Durkin T. Inhibition of endothelial cell proliferation by type beta-transforming growth factor: interactions with acidic and basic fibroblast growth factors. Biochem Biophys Res Commun. 1986;138:476–482. - PubMed
-
- Banerjee S, Banerjee P P, Zirkin B R, Brown T R. Regional expression of transforming growth factor-alpha in rat ventral prostate during postnatal development, after androgen ablation, and after androgen replacement. Endocrinology. 1998;139:3005–3013. - PubMed
-
- Barbieri B, Balconi G, Dejana E, Donati M B. Evidence that vascular endothelial cells can induce the retraction of fibrin clots. Proc Soc Exp Biol Med. 1981;168:204–207. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
