Targeted disruption of the transition protein 2 gene affects sperm chromatin structure and reduces fertility in mice
- PMID: 11585907
- PMCID: PMC99899
- DOI: 10.1128/MCB.21.21.7243-7255.2001
Targeted disruption of the transition protein 2 gene affects sperm chromatin structure and reduces fertility in mice
Abstract
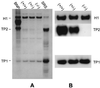
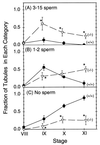
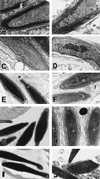
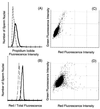
During mammalian spermiogenesis, major restructuring of chromatin takes place. In the mouse, the histones are replaced by the transition proteins, TP1 and TP2, which are in turn replaced by the protamines, P1 and P2. To investigate the role of TP2, we generated mice with a targeted deletion of its gene, Tnp2. Spermatogenesis in Tnp2 null mice was almost normal, with testis weights and epididymal sperm counts being unaffected. The only abnormality in testicular histology was a slight increase of sperm retention in stage IX to XI tubules. Epididymal sperm from Tnp2-null mice showed an increase in abnormal tail, but not head, morphology. The mice were fertile but produced small litters. In step 12 to 16 spermatid nuclei from Tnp2-null mice, there was normal displacement of histones, a compensatory translationally regulated increase in TP1 levels, and elevated levels of precursor and partially processed forms of P2. Electron microscopy revealed abnormal focal condensations of chromatin in step 11 to 13 spermatids and progressive chromatin condensation in later spermatids, but condensation was still incomplete in epididymal sperm. Compared to that of the wild type, the sperm chromatin of these mutants was more accessible to intercalating dyes and more susceptible to acid denaturation, which is believed to indicate DNA strand breaks. We conclude that TP2 is not a critical factor for shaping of the sperm nucleus, histone displacement, initiation of chromatin condensation, binding of protamines to DNA, or fertility but that it is necessary for maintaining the normal processing of P2 and, consequently, the completion of chromatin condensation.
Figures





Similar articles
-
Roles of transition nuclear proteins in spermiogenesis.Chromosoma. 2003 May;111(8):483-8. doi: 10.1007/s00412-002-0227-z. Epub 2003 Feb 6. Chromosoma. 2003. PMID: 12743712 Review.
-
Abnormal spermatogenesis and reduced fertility in transition nuclear protein 1-deficient mice.Proc Natl Acad Sci U S A. 2000 Apr 25;97(9):4683-8. doi: 10.1073/pnas.97.9.4683. Proc Natl Acad Sci U S A. 2000. PMID: 10781074 Free PMC article.
-
Abnormalities and reduced reproductive potential of sperm from Tnp1- and Tnp2-null double mutant mice.Biol Reprod. 2004 Oct;71(4):1220-9. doi: 10.1095/biolreprod.104.029363. Epub 2004 Jun 9. Biol Reprod. 2004. PMID: 15189834
-
Transition nuclear proteins are required for normal chromatin condensation and functional sperm development.Genesis. 2004 Apr;38(4):200-13. doi: 10.1002/gene.20019. Genesis. 2004. PMID: 15083521
-
Sperm chromatin protamination: an endocrine perspective.Protein Pept Lett. 2011 Aug;18(8):786-801. doi: 10.2174/092986611795714005. Protein Pept Lett. 2011. PMID: 21443490 Review.
Cited by
-
Alteration of poly(ADP-ribose) metabolism affects murine sperm nuclear architecture by impairing pericentric heterochromatin condensation.Chromosoma. 2013 Aug;122(4):319-35. doi: 10.1007/s00412-013-0416-y. Epub 2013 Jun 1. Chromosoma. 2013. PMID: 23729169 Free PMC article.
-
Roles of transition nuclear proteins in spermiogenesis.Chromosoma. 2003 May;111(8):483-8. doi: 10.1007/s00412-002-0227-z. Epub 2003 Feb 6. Chromosoma. 2003. PMID: 12743712 Review.
-
Polar nuclear localization of H1T2, a histone H1 variant, required for spermatid elongation and DNA condensation during spermiogenesis.Proc Natl Acad Sci U S A. 2005 Feb 22;102(8):2808-13. doi: 10.1073/pnas.0406060102. Epub 2005 Feb 14. Proc Natl Acad Sci U S A. 2005. PMID: 15710904 Free PMC article.
-
Jmjd1a demethylase-regulated histone modification is essential for cAMP-response element modulator-regulated gene expression and spermatogenesis.J Biol Chem. 2010 Jan 22;285(4):2758-70. doi: 10.1074/jbc.M109.066845. Epub 2009 Nov 12. J Biol Chem. 2010. PMID: 19910458 Free PMC article.
-
Mouse models in male fertility research.Asian J Androl. 2011 Jan;13(1):139-51. doi: 10.1038/aja.2010.101. Epub 2010 Nov 8. Asian J Androl. 2011. PMID: 21057516 Free PMC article. Review.
References
-
- Alfonso P J, Kistler W S. Immunohistochemical localization of spermatid nuclear transition protein 2 in the testes of rats and mice. Biol Reprod. 1993;48:522–529. - PubMed
-
- Arango N A, Lovell-Badge R, Behringer R R. Targeted mutagenesis of the endogenous mouse Mis gene promoter: in vivo definition of genetic pathways of vertebrate sexual development. Cell. 1999;99:409–419. - PubMed
-
- Aravindan G R, Bjordahl J, Jost L K, Evenson D P. Susceptibility of human sperm to in situ DNA denaturation is strongly correlated with DNA strand breaks identified by single-cell electrophoresis. Exp Cell Res. 1997;236:231–237. - PubMed
-
- Balhorn R, Gledhill B L, Wyrobek A J. Mouse sperm in chromatin proteins: quantitative isolation and partial characterization. Biochemistry. 1977;16:4074–4080. - PubMed
-
- Balhorn R, Reed S, Tanphaichitr N. Aberrant protamine 1/protamine 2 ratios in sperm of infertile human males. Experientia. 1988;44:52–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
