The transcriptional repressor REST determines the cell-specific expression of the human MAPK8IP1 gene encoding IB1 (JIP-1)
- PMID: 11585908
- PMCID: PMC99900
- DOI: 10.1128/MCB.21.21.7256-7267.2001
The transcriptional repressor REST determines the cell-specific expression of the human MAPK8IP1 gene encoding IB1 (JIP-1)
Abstract
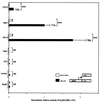
Islet-brain 1 (IB1) is the human and rat homologue of JIP-1, a scaffold protein interacting with the c-Jun amino-terminal kinase (JNK). IB1 expression is mostly restricted to the endocrine pancreas and to the central nervous system. Herein, we explored the transcriptional mechanism responsible for this preferential islet and neuronal expression of IB1. A 731-bp fragment of the 5' regulatory region of the human MAPK8IP1 gene was isolated from a human BAC library and cloned upstream of a luciferase reporter gene. This construct drove high transcriptional activity in both insulin-secreting and neuron-like cells but not in unrelated cell lines. Sequence analysis of this promoter region revealed the presence of a neuron-restrictive silencer element (NRSE) known to bind repressor zinc finger protein REST. This factor is not expressed in insulin-secreting and neuron-like cells. By mobility shift assay, we confirmed that REST binds to the NRSE present in the IB1 promoter. Once transiently transfected in beta-cell lines, the expression vector encoding REST repressed IB1 transcriptional activity. The introduction of a mutated NRSE in the 5' regulating region of the IB1 gene abolished the repression activity driven by REST in insulin-secreting beta cells and relieved the low transcriptional activity of IB1 observed in unrelated cells. Moreover, transfection in non-beta and nonneuronal cell lines of an expression vector encoding REST lacking its transcriptional repression domain relieved IB1 promoter activity. Last, the REST-mediated repression of IB1 could be abolished by trichostatin A, indicating that deacetylase activity is required to allow REST repression. Taken together, these data establish a critical role for REST in the control of the tissue-specific expression of the human IB1 gene.
Figures









References
-
- Atouf F, Czernichow P, Scharfmann R. Expression of neuronal traits in pancreatic beta cells. Implication of neuron-restrictive silencing factor/repressor element silencing transcription factor, a neuron-restrictive silencer. J Biol Chem. 1997;272:1929–1934. - PubMed
-
- Bonny C, Nicod P, Waeber G. IB1, a JIP-1-related nuclear protein present in insulin-secreting cells. J Biol Chem. 1998;273:1843–1846. - PubMed
-
- Bonny C, Oberson A, Steinmann M, Schorderet D F, Nicod P, Waeber G. IB1 reduces cytokine-induced apoptosis of insulin-secreting cells. J Biol Chem. 2000;275:16466–16472. - PubMed
-
- Bonny C, Thompson N, Nicod P, Waeber G. Pancreatic-specific expression of the glucose transporter type 2 gene: identification of cis-elements and islet-specific trans-acting factors. Mol Endocrinol. 1995;9:1413–1426. - PubMed
-
- Chen Z F, Paquette A J, Anderson D J. NRSF/REST is required in vivo for repression of multiple neuronal target genes during embryogenesis. Nat Genet. 1998;20:136–142. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
