Bid, a widely expressed proapoptotic protein of the Bcl-2 family, displays lipid transfer activity
- PMID: 11585909
- PMCID: PMC99901
- DOI: 10.1128/MCB.21.21.7268-7276.2001
Bid, a widely expressed proapoptotic protein of the Bcl-2 family, displays lipid transfer activity
Abstract
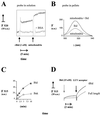
Bid is an abundant proapoptotic protein of the Bcl-2 family that is crucial for the induction of death receptor-mediated apoptosis in primary tissues such as liver. Bid action has been proposed to involve the relocation of its truncated form, tBid, to mitochondria to facilitate the release of apoptogenic cytochrome c. The mechanism of Bid relocation to mitochondria was unclear. We report here novel biochemical evidence indicating that Bid has lipid transfer activity between mitochondria and other intracellular membranes, thereby explaining its dynamic relocation to mitochondria. First, physiological concentrations of phospholipids such as phosphatidic acid and phosphatidylglycerol induced an accumulation of full-length Bid in mitochondria when incubated with light membranes enriched in endoplasmic reticulum. Secondly, native and recombinant Bid, as well as tBid, displayed lipid transfer activity under the same conditions and at the same nanomolar concentrations leading to mitochondrial relocation and release of cytochrome c. Thus, Bid is likely to be involved in the transport and recycling of mitochondrial phospholipids. We discuss how this new role of Bid may relate to its proapoptotic action.
Figures


Similar articles
-
Pro-apoptotic Bid induces membrane perturbation by inserting selected lysolipids into the bilayer.Biochem J. 2005 Apr 1;387(Pt 1):109-18. doi: 10.1042/BJ20041389. Biochem J. 2005. PMID: 15500442 Free PMC article.
-
Proapoptotic Bid binds to monolysocardiolipin, a new molecular connection between mitochondrial membranes and cell death.Cell Death Differ. 2003 Dec;10(12):1300-9. doi: 10.1038/sj.cdd.4401306. Cell Death Differ. 2003. PMID: 12894218
-
Bax oligomerization in mitochondrial membranes requires tBid (caspase-8-cleaved Bid) and a mitochondrial protein.Biochem J. 2002 Dec 15;368(Pt 3):915-21. doi: 10.1042/BJ20020972. Biochem J. 2002. PMID: 12193163 Free PMC article.
-
The roles of Bid.Apoptosis. 2002 Oct;7(5):433-40. doi: 10.1023/a:1020035124855. Apoptosis. 2002. PMID: 12207176 Review.
-
Mitochondria in apoptosis: past, present and future.Biochem Soc Trans. 2004 Jun;32(Pt3):493-5. doi: 10.1042/BST0320493. Biochem Soc Trans. 2004. PMID: 15157169 Review.
Cited by
-
Sexual Differences in Cell Loss during the Post-Hatch Development of Song Control Nuclei in the Bengalese Finch.PLoS One. 2015 May 4;10(5):e0125802. doi: 10.1371/journal.pone.0125802. eCollection 2015. PLoS One. 2015. PMID: 25938674 Free PMC article.
-
Phospholipids: key players in apoptosis and immune regulation.Molecules. 2009 Nov 30;14(12):4892-914. doi: 10.3390/molecules14124892. Molecules. 2009. PMID: 20032867 Free PMC article. Review.
-
Pro-apoptotic Bid induces membrane perturbation by inserting selected lysolipids into the bilayer.Biochem J. 2005 Apr 1;387(Pt 1):109-18. doi: 10.1042/BJ20041389. Biochem J. 2005. PMID: 15500442 Free PMC article.
-
Bid-cardiolipin interaction at mitochondrial contact site contributes to mitochondrial cristae reorganization and cytochrome C release.Mol Biol Cell. 2004 Jul;15(7):3061-72. doi: 10.1091/mbc.e03-12-0864. Epub 2004 Apr 23. Mol Biol Cell. 2004. PMID: 15107464 Free PMC article.
-
NME4/nucleoside diphosphate kinase D in cardiolipin signaling and mitophagy.Lab Invest. 2018 Feb;98(2):228-232. doi: 10.1038/labinvest.2017.113. Epub 2017 Oct 16. Lab Invest. 2018. PMID: 29035377 Review.
References
-
- Atsumi G, Tajima M, Hadano A, Nakatani Y, Murakami M, Kudo I. Fas-induced arachidonic acid release is mediated by Ca2+-independent phospholipase A2 but not cytosolic phospholipase A2, which undergoes proteolytic inactivation. J Biol Chem. 1998;273:13870–13877. - PubMed
-
- Bai J, Pagano R E. Measurement of spontaneous transfer and transbilayer movement of BODIPY-labeled lipids in lipid vesicles. Biochemistry. 1997;36:8840–8848. - PubMed
-
- Bogin L, Papa M Z, Polak-Charcon S, Degani H. TNF-induced modulations of phospholipid metabolism in human breast cancer cells. Biochim Biophys Acta. 1998;1392:217–232. - PubMed
-
- Bossy-Wetzel E, Green D R. Caspases induce cytochrome c release from mitochondria by activating cytosolic factors. J Biol Chem. 1999;274:17484–17490. - PubMed
-
- Chakraborty T R, Vancura A, Balija V, Haldar D. Phosphatidic acid synthesis in mitochondria. J Biol Chem. 1999;274:29786–29790. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
