Distinct RNP complexes of shuttling hnRNP proteins with pre-mRNA and mRNA: candidate intermediates in formation and export of mRNA
- PMID: 11585913
- PMCID: PMC99905
- DOI: 10.1128/MCB.21.21.7307-7319.2001
Distinct RNP complexes of shuttling hnRNP proteins with pre-mRNA and mRNA: candidate intermediates in formation and export of mRNA
Abstract
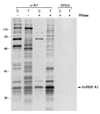
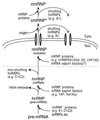
Nascent pre-mRNAs associate with hnRNP proteins in hnRNP complexes, the natural substrates for mRNA processing. Several lines of evidence indicate that hnRNP complexes undergo substantial remodeling during mRNA formation and export. Here we report the isolation of three distinct types of pre-mRNP and mRNP complexes from HeLa cells associated with hnRNP A1, a shuttling hnRNP protein. Based on their RNA and protein compositions, these complexes are likely to represent distinct stages in the nucleocytoplasmic shuttling pathway of hnRNP A1 with its bound RNAs. In the cytoplasm, A1 is associated with its nuclear import receptor (transportin), the cytoplasmic poly(A)-binding protein, and mRNA. In the nucleus, A1 is found in two distinct types of complexes that are differently associated with nuclear structures. One class contains pre-mRNA and mRNA and is identical to previously described hnRNP complexes. The other class behaves as freely diffusible nuclear mRNPs (nmRNPs) at late nuclear stages of maturation and possibly associated with nuclear mRNA export. These nmRNPs differ from hnRNPs in that while they contain shuttling hnRNP proteins, the mRNA export factor REF, and mRNA, they do not contain nonshuttling hnRNP proteins or pre-mRNA. Importantly, nmRNPs also contain proteins not found in hnRNP complexes. These include the alternatively spliced isoforms D01 and D02 of the hnRNP D proteins, the E0 isoform of the hnRNP E proteins, and LRP130, a previously reported protein with unknown function that appears to have a novel type of RNA-binding domain. The characteristics of these complexes indicate that they result from RNP remodeling associated with mRNA maturation and delineate specific changes in RNP protein composition during formation and transport of mRNA in vivo.
Figures
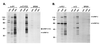




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
