Orphanin FQ/nociceptin attenuates the development of morphine tolerance in rats
- PMID: 11588106
- PMCID: PMC1572978
- DOI: 10.1038/sj.bjp.0704279
Orphanin FQ/nociceptin attenuates the development of morphine tolerance in rats
Abstract
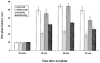
1. Recent evidence from studies in mice lacking the opioid receptor-like (ORL-1) receptor and from experiments using antibodies raised against orphanin FQ/nociceptin (OFQ/N) suggest that this peptide may be involved in morphine tolerance. In the present study we sought to investigate if administration of exogenous OFQ/N would modulate the development of tolerance to the antinociceptive effect of morphine. 2. Rats were treated for 3 days with either saline or morphine (10 mg kg(-1), s.c.) followed, 15 and 75 min later, by two intracerebroventricular injections of either artificial cerebrospinal fluid (aCSF) or OFQ/N. The dose of OFQ/N was doubled each day (7.5, 15, 30 nmol). On day 4, rats were tested on a hot plate apparatus before and 30, 60 and 90 min after morphine administration. 3. Repeated OFQ/N treatment did not affect basal nociceptive responses or morphine-induced antinociception. However, the same treatment significantly attenuated the development of morphine tolerance. 4. Since learning and memory could contribute to the development of morphine tolerance, in subsequent studies, we examined the effect of OFQ/N administered in the CA3 region of the hippocampus, where OFQ/N has been shown to block LTP and impair spatial memory. A greater attenuation of morphine tolerance with no alteration of baseline hot plate latency or morphine-induced antinociception was observed when OFQ/N was administered in this area of the rat brain. 5. Taken together, our results demonstrate that OFQ/N may act in the hippocampus to attenuate morphine tolerance.
Figures
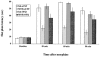
Similar articles
-
Changes in nociceptin/orphanin FQ levels in rat brain regions after acute and chronic cannabinoid treatment in conjunction with the development of antinociceptive tolerance.Fundam Clin Pharmacol. 2016 Dec;30(6):537-548. doi: 10.1111/fcp.12215. Epub 2016 Jul 14. Fundam Clin Pharmacol. 2016. PMID: 27371029
-
Tolerance develops to the inhibitory effect of orphanin FQ on morphine-induced antinociception in the rat.Neuroreport. 1999 Jan 18;10(1):103-6. doi: 10.1097/00001756-199901180-00020. Neuroreport. 1999. PMID: 10094142
-
Antinociceptive and morphine modulatory actions of spinal orphanin FQ.Can J Physiol Pharmacol. 1998 Mar;76(3):314-24. Can J Physiol Pharmacol. 1998. PMID: 9673795
-
Nocistatin: a novel neuropeptide encoded by the gene for the nociceptin/orphanin FQ precursor.Peptides. 2000 Jul;21(7):1101-9. doi: 10.1016/s0196-9781(00)00247-3. Peptides. 2000. PMID: 10998544 Review.
-
The biology of Nociceptin/Orphanin FQ (N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence.Pharmacol Ther. 2014 Mar;141(3):283-99. doi: 10.1016/j.pharmthera.2013.10.011. Epub 2013 Nov 1. Pharmacol Ther. 2014. PMID: 24189487 Free PMC article. Review.
Cited by
-
Involvement of the N/OFQ-NOP system in rat morphine antinociceptive tolerance: Are astrocytes the crossroad?Eur J Pharmacol. 2018 Mar 15;823:79-86. doi: 10.1016/j.ejphar.2018.01.039. Epub 2018 Jan 31. Eur J Pharmacol. 2018. PMID: 29378191 Free PMC article.
-
Endogenous opioid systems alterations in pain and opioid use disorder.Front Syst Neurosci. 2022 Oct 19;16:1014768. doi: 10.3389/fnsys.2022.1014768. eCollection 2022. Front Syst Neurosci. 2022. PMID: 36341476 Free PMC article. Review.
-
The nociceptin/orphanin FQ receptor (NOP) as a target for drug abuse medications.Curr Top Med Chem. 2011;11(9):1151-6. doi: 10.2174/156802611795371341. Curr Top Med Chem. 2011. PMID: 21050175 Free PMC article. Review.
-
Molecular and cellular mechanisms of the age-dependency of opioid analgesia and tolerance.Mol Pain. 2012 May 21;8:38. doi: 10.1186/1744-8069-8-38. Mol Pain. 2012. PMID: 22612909 Free PMC article. Review.
-
Current and Future Therapeutic Options in Pain Management: Multi-mechanistic Opioids Involving Both MOR and NOP Receptor Activation.CNS Drugs. 2022 Jun;36(6):617-632. doi: 10.1007/s40263-022-00924-2. Epub 2022 May 26. CNS Drugs. 2022. PMID: 35616826 Free PMC article. Review.
References
-
- ARDEN J.R., SEGREDO V., WANG Z., LAMEH J., SADEE W. Phosphorylation and agonist-specific intracellular trafficking of an epitope-tagged mu-opioid receptor expressed in HEK 293 cells. J. Neurochem. 1995;65:1636–1645. - PubMed
-
- BECKER C.M., POHL M., THIEBOT M.H., COLLIN E., HAMON M., CESSELIN F., BENOLIEL J.J. Delta-opioid receptor-mediated increase in cortical extracellular levels of cholecystokinin-like material by subchronic morphine in rats. Neuropharmacology. 2000;39:161–171. - PubMed
-
- BERNSTEIN M.A., WELCH S.P. mu-Opioid receptor down-regulation and cAMP-dependent protein kinase phosphorylation in a mouse model of chronic morphine tolerance. Mol. Brain Res. 1998;55:237–242. - PubMed
-
- CICCOCIOPPO R., ANGELETTI S., SANNA P.P., WEISS F., MASSI M. Effect of nociceptin/orphanin FQ on the rewarding properties of morphine. Eur. J. Pharmacol. 2000;404:153–159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

