The KATP channel opener diazoxide protects cardiac myocytes during metabolic inhibition without causing mitochondrial depolarization or flavoprotein oxidation
- PMID: 11588107
- PMCID: PMC1572983
- DOI: 10.1038/sj.bjp.0704289
The KATP channel opener diazoxide protects cardiac myocytes during metabolic inhibition without causing mitochondrial depolarization or flavoprotein oxidation
Abstract
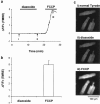
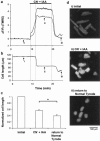
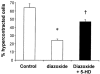
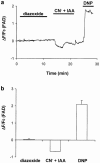
1. The K(ATP) channel opener diazoxide has been proposed to protect cardiac muscle against ischaemia by opening mitochondrial K(ATP) channels to depolarize the mitochondrial membrane potential, DeltaPsi(m). We have used the fluorescent dye TMRE to measure DeltaPsi(m) in adult rat freshly isolated cardiac myocytes exposed to diazoxide and metabolic inhibition. 2. Diazoxide, at concentrations that are highly cardioprotective (100 or 200 microM), caused no detectable increase in TMRE fluorescence (n=27 cells). However, subsequent application of the protonophore FCCP, which should collapse DeltaPsi(m), led to large increases in TMRE fluorescence (>300%). 3. Metabolic inhibition (MI: 2 mM NaCN+1 mM iodoacetic acid (IAA) led to an immediate partial depolarization of DeltaPsi(m), followed after a few minutes delay by complete depolarization which was correlated with rigor contracture. Removal of metabolic inhibition led to abrupt mitochondrial repolarization followed in many cells by hypercontracture, indicated by cell rounding and loss of striated appearance. 4. Prior application of diazoxide (100 microM) reduced the number of cells that hypercontracted after metabolic inhibition from 63.7+/-4.7% to 24.2+/-1.8% (P< 0.0001). 5-hydroxydeanoate (100 microM) reduced the protection of diazoxide (46.8+/-2.7% cells hypercontracted, P< 0.0001 vs diazoxide alone). 5. Diazoxide caused no detectable change in flavoprotein autofluorescence (n=26 cells). 6. Our results suggest that mitochondrial depolarization and flavoprotein oxidation are not inevitable consequences of diazoxide application in intact cardiac myocytes, and that they are also not essential components of the mechanism by which it causes protection.
Figures

Similar articles
-
P-1075 exerts diverse modulatory effects on mitochondrial ATP-sensitive K+ channels in rabbit ventricular myocytes.J Cardiovasc Pharmacol. 2006 Feb;47(2):165-8. doi: 10.1097/01.fjc.0000199224.40799.a4. J Cardiovasc Pharmacol. 2006. PMID: 16495751
-
FCCP is cardioprotective at concentrations that cause mitochondrial oxidation without detectable depolarisation.Cardiovasc Res. 2006 Nov 1;72(2):322-30. doi: 10.1016/j.cardiores.2006.08.006. Epub 2006 Aug 16. Cardiovasc Res. 2006. PMID: 16979603
-
Pharmacological and histochemical distinctions between molecularly defined sarcolemmal KATP channels and native cardiac mitochondrial KATP channels.Mol Pharmacol. 1999 Jun;55(6):1000-5. Mol Pharmacol. 1999. PMID: 10347240
-
Preconditioning with diazoxide prevents reoxygenation-induced rigor-type hypercontracture.J Mol Cell Cardiol. 2010 Jan;48(1):270-6. doi: 10.1016/j.yjmcc.2009.04.013. Epub 2009 May 4. J Mol Cell Cardiol. 2010. PMID: 19406125
-
Mitochondrial Ca2+-activated K+ channels in cardiac myocytes: a mechanism of the cardioprotective effect and modulation by protein kinase A.Circulation. 2005 Jan 18;111(2):198-203. doi: 10.1161/01.CIR.0000151099.15706.B1. Epub 2004 Dec 27. Circulation. 2005. PMID: 15623543
Cited by
-
Inhibition of mitochondrial membrane permeability as a putative pharmacological target for cardioprotection.Curr Med Chem. 2009;16(33):4382-98. doi: 10.2174/092986709789712871. Curr Med Chem. 2009. PMID: 19835566 Free PMC article. Review.
-
K(ATP) channel-independent targets of diazoxide and 5-hydroxydecanoate in the heart.J Physiol. 2002 Aug 1;542(Pt 3):735-41. doi: 10.1113/jphysiol.2002.023960. J Physiol. 2002. PMID: 12154175 Free PMC article.
-
Pharmacological activation of plasma-membrane KATP channels reduces reoxygenation-induced Ca(2+) overload in cardiac myocytes via modulation of the diastolic membrane potential.Br J Pharmacol. 2004 Mar;141(6):1059-67. doi: 10.1038/sj.bjp.0705702. Epub 2004 Mar 1. Br J Pharmacol. 2004. PMID: 14993099 Free PMC article.
-
The role of mitochondria in protection of the heart by preconditioning.Biochim Biophys Acta. 2007 Aug;1767(8):1007-31. doi: 10.1016/j.bbabio.2007.05.008. Epub 2007 Jun 2. Biochim Biophys Acta. 2007. PMID: 17631856 Free PMC article. Review.
-
Slowly activating voltage-gated potassium current potentiation by ML277 is a novel cardioprotective intervention.PNAS Nexus. 2023 May 10;2(5):pgad156. doi: 10.1093/pnasnexus/pgad156. eCollection 2023 May. PNAS Nexus. 2023. PMID: 37234204 Free PMC article.
References
-
- COHEN M.V., BAINES C.P., DOWNEY J.M. Ischemic preconditioning: From adenosine receptor to KATP channel. Ann. Rev. Physiol. 2000;62:79–109. - PubMed
-
- EMAUS R.K., GRUNWALD R., LEMASTERS J.J. Rhodamine 123 as a probe of transmembrane potential in isolated rat-liver mitochondria: spectra and metabolic properties. Biochim. Biophys. Acta. 1986;850:436–448. - PubMed
-
- FRYER R.M., HSU A.K., NAGASE H., GROSS G.J. Opioid-induced cardioprotection against myocardial infarction and arrhythmias: mitochondrial versus sarcolemmal ATP-sensitive potassium channels. J. Pharmacol. Exp. Ther. 2000;294:451–457. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

