Therapeutic efficacy in experimental polyarthritis of viral-driven enkephalin overproduction in sensory neurons
- PMID: 11588161
- PMCID: PMC6763863
- DOI: 10.1523/JNEUROSCI.21-20-07881.2001
Therapeutic efficacy in experimental polyarthritis of viral-driven enkephalin overproduction in sensory neurons
Abstract
Rheumatoid arthritis is characterized by erosive inflammation of the joints, new bone proliferation, and ankylosis, leading to severely reduced locomotion and intense chronic pain. In a model of this disease, adjuvant-induced polyarthritis in the rat, neurons involved in pain transmission and control undergo plastic changes, especially at the spinal level. These changes affect notably neurons that contain opioids, such as enkephalins deriving from preproenkephalin A (PA) precursor protein. Using recombinant herpes simplex virus containing rat PA cDNA, we enhanced enkephalin synthesis in sensory neurons of polyarthritic rats. This treatment markedly improved locomotion and reduced hyperalgesia. Furthermore, the progression of bone destruction slowed down, which is the most difficult target to reach in the treatment of patients suffering from arthritis. These data demonstrate the therapeutic efficacy of enkephalin overproduction in a model of systemic inflammatory and painful chronic disorder.
Figures
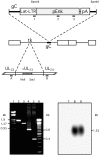
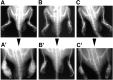
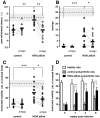
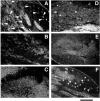

References
-
- Antunes-Bras JM, Epstein AL, Bourgoin S, Hamon M, Cesselin F, Pohl M. Herpes simplex virus1-mediated transfer of preproenkephalin A in rat dorsal root ganglia. J Neurochem. 1998;70:1299–1303. - PubMed
-
- Antunes-Bras JM, Becker C, Bourgoin S, Lombard MC, Cesselin F, Hamon M, Pohl M. Met-enkephalin is preferentially transported to the peripheral processes of primary afferent fibres in both normal and proenkephalin A overexpressing rats. Neuroscience. 2001;103:1073–1083. - PubMed
-
- Bjurholm A, Kreibergs A, Brodin E, Schultzberg M. Substance P- and CGRP-immunoreactive nerves in bone. Peptides. 1988;9:165–171. - PubMed
-
- Cain CK, Francis JM, Plone MA, Emerich DF, Lindner MD. Pain-related disability and effects of chronic morphine in the adjuvant-induced arthritis model of chronic pain. Physiol Behav. 1997;62:199–205. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
