Estimation of quantal size and number of functional active zones at the calyx of Held synapse by nonstationary EPSC variance analysis
- PMID: 11588162
- PMCID: PMC6763848
- DOI: 10.1523/JNEUROSCI.21-20-07889.2001
Estimation of quantal size and number of functional active zones at the calyx of Held synapse by nonstationary EPSC variance analysis
Abstract
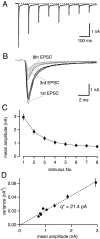
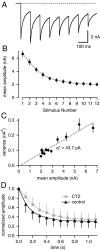
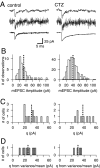
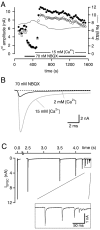
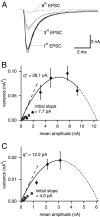
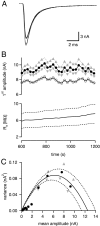
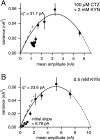
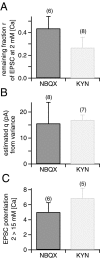
At the large excitatory calyx of Held synapse, the quantal size during an evoked EPSC and the number of active zones contributing to transmission are not known. We developed a nonstationary variant of EPSC fluctuation analysis to determine these quantal parameters. AMPA receptor-mediated EPSCs were recorded in slices of young (postnatal 8-10 d) rats after afferent fiber stimulation, delivered in trains to induce synaptic depression. The means and the variances of EPSC amplitudes were calculated across trains for each stimulus number. During 10 Hz trains at 2 mm Ca(2+) concentration ([Ca(2+)]), we found linear EPSC variance-mean relationships, with a slope that was in good agreement with the quantal size obtained from amplitude distributions of spontaneous miniature EPSCs. At high release probability with 10 or 15 mm [Ca(2+)], competitive antagonists were used to partially block EPSCs. Under these conditions, the EPSC variance-mean plots could be fitted with parabolas, giving estimates of quantal size and of the binomial parameter N. With the rapidly dissociating antagonist kynurenic acid, quantal sizes were larger than with a slowly dissociating antagonist, suggesting that the effective glutamate concentration was increased at high release probability. Considering the possibility of multivesicular release and moderate saturation of postsynaptic AMPA receptors, we conclude that the binomial parameter N (637 +/- 117; mean +/- SEM) represents an upper limit estimate of the number of functional active zones. We estimate that during normal synaptic transmission, the probability of vesicle fusion at single active zones is in the range of 0.25-0.4.
Figures
Similar articles
-
Separation of presynaptic and postsynaptic contributions to depression by covariance analysis of successive EPSCs at the calyx of Held synapse.J Neurosci. 2002 Feb 1;22(3):728-39. doi: 10.1523/JNEUROSCI.22-03-00728.2002. J Neurosci. 2002. PMID: 11826102 Free PMC article.
-
Locus of frequency-dependent depression identified with multiple-probability fluctuation analysis at rat climbing fibre-Purkinje cell synapses.J Physiol. 1998 Aug 1;510 ( Pt 3)(Pt 3):881-902. doi: 10.1111/j.1469-7793.1998.881bj.x. J Physiol. 1998. PMID: 9660900 Free PMC article.
-
Developmental increase in vesicular glutamate content does not cause saturation of AMPA receptors at the calyx of Held synapse.J Neurosci. 2003 May 1;23(9):3633-8. doi: 10.1523/JNEUROSCI.23-09-03633.2003. J Neurosci. 2003. PMID: 12736334 Free PMC article.
-
Estimation of quantal parameters at the calyx of Held synapse.Neurosci Res. 2002 Dec;44(4):343-56. doi: 10.1016/s0168-0102(02)00174-8. Neurosci Res. 2002. PMID: 12445623 Review.
-
Quantal analysis of excitatory postsynaptic currents at the hippocampal mossy fiber-CA3 pyramidal cell synapse.Adv Second Messenger Phosphoprotein Res. 1994;29:235-60. doi: 10.1016/s1040-7952(06)80019-4. Adv Second Messenger Phosphoprotein Res. 1994. PMID: 7848714 Review.
Cited by
-
Time Course of Activity-Dependent Changes in Auditory Nerve Synapses Reveals Multiple Underlying Cellular Mechanisms.J Neurosci. 2022 Mar 23;42(12):2492-2502. doi: 10.1523/JNEUROSCI.1583-21.2022. Epub 2022 Feb 18. J Neurosci. 2022. PMID: 35181597 Free PMC article.
-
Structural and Functional Plasticity in the Dorsolateral Geniculate Nucleus of Mice following Bilateral Enucleation.Neuroscience. 2022 Apr 15;488:44-59. doi: 10.1016/j.neuroscience.2022.01.029. Epub 2022 Feb 4. Neuroscience. 2022. PMID: 35131394 Free PMC article.
-
The timing of phasic transmitter release is Ca2+-dependent and lacks a direct influence of presynaptic membrane potential.Proc Natl Acad Sci U S A. 2003 Dec 9;100(25):15200-5. doi: 10.1073/pnas.2433276100. Epub 2003 Nov 20. Proc Natl Acad Sci U S A. 2003. PMID: 14630950 Free PMC article.
-
Quantitative analysis of calcium-dependent vesicle recruitment and its functional role at the calyx of Held synapse.J Neurosci. 2007 Dec 26;27(52):14286-98. doi: 10.1523/JNEUROSCI.4122-07.2007. J Neurosci. 2007. PMID: 18160636 Free PMC article.
-
Munc18-1 is a dynamically regulated PKC target during short-term enhancement of transmitter release.Elife. 2014 Feb 11;3:e01715. doi: 10.7554/eLife.01715. Elife. 2014. PMID: 24520164 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
