Gating properties of Na(v)1.7 and Na(v)1.8 peripheral nerve sodium channels
- PMID: 11588164
- PMCID: PMC6763856
- DOI: 10.1523/JNEUROSCI.21-20-07909.2001
Gating properties of Na(v)1.7 and Na(v)1.8 peripheral nerve sodium channels
Abstract
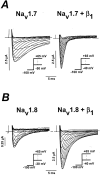
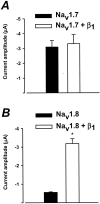
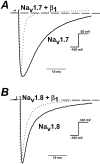
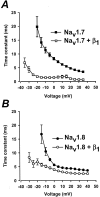
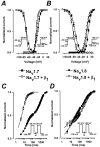
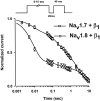
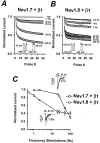
Several distinct components of voltage-gated sodium current have been recorded from native dorsal root ganglion (DRG) neurons that display differences in gating and pharmacology. This study compares the electrophysiological properties of two peripheral nerve sodium channels that are expressed selectively in DRG neurons (Na(v)1.7 and Na(v)1.8). Recombinant Na(v)1.7 and Na(v)1.8 sodium channels were coexpressed with the auxiliary beta(1) subunit in Xenopus oocytes. In this system coexpression of the beta(1) subunit with Na(v)1.7 and Na(v)1.8 channels results in more rapid inactivation, a shift in midpoints of steady-state activation and inactivation to more hyperpolarizing potentials, and an acceleration of recovery from inactivation. The coinjection of beta(1) subunit also significantly increases the expression of Na(v)1.8 by sixfold but has no effect on the expression of Na(v)1.7. In addition, a great percentage of Na(v)1.8+beta(1) channels is observed to enter rapidly into the slow inactivated states, in contrast to Nav1.7+beta(1) channels. Consequently, the rapid entry into slow inactivation is believed to cause a frequency-dependent reduction of Na(v)1.8+beta(1) channel amplitudes, seen during repetitive pulsing between 1 and 2 Hz. However, at higher frequencies (>20 Hz) Na(v)1.8+beta(1) channels reach a steady state to approximately 42% of total current. The presence of this steady-state sodium channel activity, coupled with the high activation threshold (V(0.5) = -3.3 mV) of Na(v)1.8+beta(1), could enable the nociceptive fibers to fire spontaneously after nerve injury.
Figures
Similar articles
-
Regulation of Nav1.6 and Nav1.8 peripheral nerve Na+ channels by auxiliary β-subunits.J Neurophysiol. 2011 Aug;106(2):608-19. doi: 10.1152/jn.00107.2011. Epub 2011 May 11. J Neurophysiol. 2011. PMID: 21562192 Free PMC article.
-
Nav1.3 sodium channels: rapid repriming and slow closed-state inactivation display quantitative differences after expression in a mammalian cell line and in spinal sensory neurons.J Neurosci. 2001 Aug 15;21(16):5952-61. doi: 10.1523/JNEUROSCI.21-16-05952.2001. J Neurosci. 2001. PMID: 11487618 Free PMC article.
-
The sodium channel {beta}3-subunit induces multiphasic gating in NaV1.3 and affects fast inactivation via distinct intracellular regions.J Biol Chem. 2010 Oct 22;285(43):33404-33412. doi: 10.1074/jbc.M110.114058. Epub 2010 Jul 30. J Biol Chem. 2010. PMID: 20675377 Free PMC article.
-
The Na(V)1.7 sodium channel: from molecule to man.Nat Rev Neurosci. 2013 Jan;14(1):49-62. doi: 10.1038/nrn3404. Epub 2012 Dec 12. Nat Rev Neurosci. 2013. PMID: 23232607 Review.
-
The roles of sodium channels in nociception: Implications for mechanisms of pain.Pain. 2007 Oct;131(3):243-257. doi: 10.1016/j.pain.2007.07.026. Epub 2007 Sep 4. Pain. 2007. PMID: 17766042 Free PMC article. Review.
Cited by
-
Conotoxins targeting neuronal voltage-gated sodium channel subtypes: potential analgesics?Toxins (Basel). 2012 Nov 8;4(11):1236-60. doi: 10.3390/toxins4111236. Toxins (Basel). 2012. PMID: 23202314 Free PMC article. Review.
-
Differential expression of sodium channel β subunits in dorsal root ganglion sensory neurons.J Biol Chem. 2012 Apr 27;287(18):15044-53. doi: 10.1074/jbc.M111.333740. Epub 2012 Mar 9. J Biol Chem. 2012. PMID: 22408255 Free PMC article.
-
Voltage-gated Na+ channels: multiplicity of expression, plasticity, functional implications and pathophysiological aspects.Eur Biophys J. 2004 May;33(3):180-93. doi: 10.1007/s00249-004-0389-0. Epub 2004 Feb 12. Eur Biophys J. 2004. PMID: 14963621 Review.
-
Sodium channel beta2 subunits regulate tetrodotoxin-sensitive sodium channels in small dorsal root ganglion neurons and modulate the response to pain.J Neurosci. 2006 Jul 26;26(30):7984-94. doi: 10.1523/JNEUROSCI.2211-06.2006. J Neurosci. 2006. PMID: 16870743 Free PMC article.
-
Differential modulation of Nav1.7 and Nav1.8 peripheral nerve sodium channels by the local anesthetic lidocaine.Br J Pharmacol. 2004 Jun;142(3):576-84. doi: 10.1038/sj.bjp.0705796. Epub 2004 May 17. Br J Pharmacol. 2004. PMID: 15148257 Free PMC article.
References
-
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. - PubMed
-
- Arbuckle JB, Docherty RJ. Expression of tetrodotoxin-resistant sodium channels in capsaicin-sensitive dorsal root ganglion neurons of adult rats. Neurosci Lett. 1995;185:70–73. - PubMed
-
- Auld VJ, Goldin AL, Krafte DS, Marshall J, Dunn JM, Catterall WA, Lester HA, Davidson N, Dunn RJ. A rat brain Na+ channel α subunit with novel gating properties. Neuron. 1988;1:449–461. - PubMed
-
- Bennett PB, Jr, Makita N, George AL., Jr A molecular basis for gating mode transitions in human skeletal muscle Na+ channels. FEBS Lett. 1993;326:21–24. - PubMed
-
- Black JA, Dib-Hajj S, McNabola K, Jeste S, Rizzo MA, Kocsis JD, Waxman SG. Spinal sensory neurons express multiple sodium channel α subunit mRNAs. Brain Res Mol Brain Res. 1996;43:117–131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
