Protein kinase c increases the apparent affinity of the release machinery to Ca2+ by enhancing the release machinery downstream of the Ca2+ sensor
- PMID: 11588166
- PMCID: PMC6763846
- DOI: 10.1523/JNEUROSCI.21-20-07928.2001
Protein kinase c increases the apparent affinity of the release machinery to Ca2+ by enhancing the release machinery downstream of the Ca2+ sensor
Abstract
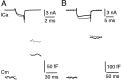
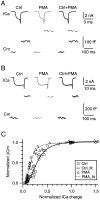
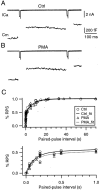
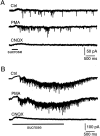
Modulation of the release probability of releasable vesicles in response to Ca(2+) influx (Prob(Ca)) is involved in mediating several forms of synaptic plasticity, including short-term depression, short-term augmentation, and potentiation induced by protein kinases. Given such an important role, however, the mechanism underlying modulation of the Prob(Ca) is unclear. We addressed this question by investigating how the activation of protein kinase C modulates the Prob(Ca) at a calyx-type nerve terminal in rat brainstem. Various lengths of step depolarization were applied to the nerve terminal to evoke different amounts of Ca(2+) currents and capacitance jumps, the latter of which reflect vesicle release. The relationship between the capacitance jump and the Ca(2+) current integral was sigmoidal and was fit well with a Hill function. The sigmoidal relationship was shifted significantly to the left during the application of the PKC activator 12-myristate 13-acetate (PMA), suggesting that PMA increases the apparent affinity of the release machinery to Ca(2+). This effect was blocked in large part by the application of the PKC inhibitor bisindolylmaleimide, suggesting that the effect is mediated mainly by the activation of PKC. We also found that PMA increased the rate of miniature EPSCs evoked by the application of hypertonic sucrose solution, which triggers release downstream of the Ca(2+) influx. Taken together, our results suggest that PKC enhances the apparent affinity of the release machinery to Ca(2+) by a mechanism downstream of the binding between Ca(2+) and its sensor. These results have provided the first example of the mechanisms underlying modulation of the Prob(Ca).
Figures
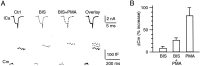


References
-
- Albillos A, Dernick G, Horstmann H, Almers W, Alvarez de Toledo G, Lindau M. The exocytotic event in chromaffin cells revealed by patch amperometry. Nature. 1997;389:509–512. - PubMed
-
- Bachoo M, Heppner T, Fiekers J, Polosa C. A role for protein kinase C in long-term potentiation of nicotinic transmission in the superior cervical ganglion of the rat. Brain Res. 1992;585:299–302. - PubMed
-
- Betz A, Ashery U, Rickmann M, Augustin I, Neher E, Sudhof TC, Rettig J, Brose N. Munc13-1 is a presynaptic phorbol ester receptor that enhances neurotransmitter release. Neuron. 1998;21:123–136. - PubMed
-
- Blackmer T, Larsen EC, Takahashi M, Martin TF, Alford S, Hamm HE. G-protein βγ subunit-mediated presynaptic inhibition: regulation of exocytotic fusion downstream of Ca2+ entry. Science. 2001;292:293–297. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
