An NMDA receptor signaling complex with protein phosphatase 2A
- PMID: 11588171
- PMCID: PMC6763850
- DOI: 10.1523/JNEUROSCI.21-20-07985.2001
An NMDA receptor signaling complex with protein phosphatase 2A
Abstract
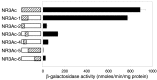
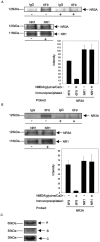
Regulation of protein phosphatase 2A (PP2A) activity and NMDA receptor (NMDAR) phosphorylation state contribute to the modulation of synaptic plasticity, yet these two mechanisms have not been functionally linked. The NMDAR subunit NR3A is equipped with a unique carboxyl domain that is different from other NMDAR subunits. We hypothesized that the NR3A C-terminal intracellular domain might serve as synaptic anchor for the phosphatase in the developing CNS. A cDNA library was screened by the yeast two-hybrid method using the NR3A carboxyl domain as the bait. The catalytic subunit of the serine-threonine PP2A was found to be associated with the NR3A carboxyl domain. Immunoprecipitation studies indicated that the NR3A subunit formed a stable complex with PP2A in the rat brain in vivo. Association of PP2A with NMDARs led to an increase in the phosphatase activity of PP2A and the dephosphorylation of serine 897 of the NMDAR subunit NR1. Stimulation of NMDARs led to the dissociation of PP2A from the complex and the reduction of PP2A activity. A peptide corresponding to the PP2A-NR3A binding domain functioned as a negative regulator of PP2A activity. These data suggest that NMDARs are allosteric modulators of PP2A, which in turn controls their phosphorylation state. The data delineate a mechanistic model of the dynamic regulation of a PP2A-NMDAR signaling complex, mediated by the interaction of NR3A and PP2A, and suggest a novel NMDAR-mediated signaling mechanism in addition to the traditional ionotropic functions of NMDARs.
Figures



References
-
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. - PubMed
-
- Das S, Sasaki YF, Rothe T, Premkumar LS, Takasu M, Crandall JE, Dikkes P, Conner DA, Rayudu PV, Cheung W, Chen HS, Lipton SA, Nakanishi N. Increased NMDA current and spine density in mice lacking the NMDA receptor subunit NR3A. Nature. 1998;393:377–381. - PubMed
-
- Faux MC, Scott JD. More on target with protein phosphorylation: conferring specificity by location. Trends Biochem Sci. 1996;21:312–315. - PubMed
-
- Fraser D, Scott JD. Modulation of ion channels: a “current” view of AKAPs. Neuron. 1999;23:423–426. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
