Induction of mossy fiber --> Ca3 long-term potentiation requires translocation of synaptically released Zn2+
- PMID: 11588174
- PMCID: PMC6763855
- DOI: 10.1523/JNEUROSCI.21-20-08015.2001
Induction of mossy fiber --> Ca3 long-term potentiation requires translocation of synaptically released Zn2+
Abstract
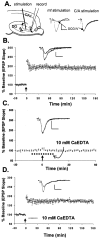
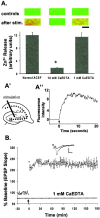
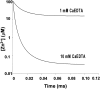
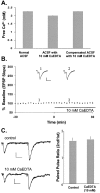
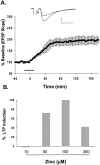
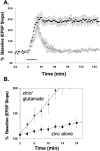
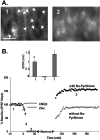
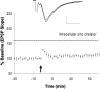
The mammalian CNS contains an abundance of chelatable Zn(2+) sequestered in the vesicles of glutamatergic terminals. These vesicles are particularly numerous in hippocampal mossy fiber synapses of the hilar and CA3 regions. Our recent observation of frequency-dependent Zn(2+) release from mossy fiber synaptic terminals and subsequent entry into postsynaptic neurons has prompted us to investigate the role of synaptically released Zn(2+) in the induction of long-term potentiation (LTP) in field CA3 of the hippocampus. The rapid removal of synaptically released Zn(2+) with the membrane-impermeable Zn(2+) chelator CaEDTA (10 mm) blocked induction of NMDA receptor-independent mossy fiber LTP by high-frequency electrical stimulation (HFS) in rat hippocampal slices. Mimicking Zn(2+) release by bath application of Zn(2+) (50-100 microm) without HFS induced a long-lasting potentiation of synaptic transmission that lasted more than 3 hr. Moreover, our experiments indicate the effects of Zn(2+) were not attributable to its interaction with extracellular membrane proteins but required its entry into presynaptic or postsynaptic neurons. Co-released glutamate is also essential for induction of LTP under physiological conditions, in part because it allows Zn(2+) entry into postsynaptic neurons. These results indicate that synaptically released Zn(2+), acting as a second messenger, is necessary for the induction of LTP at mossy fiber-->CA3 synapses of hippocampus.
Figures

References
-
- Aniksztejn L, Charton G, Ben-Ari Y. Selective release of endogenous zinc from the hippocampal mossy fibers in situ. Brain Res. 1987;404:58–64. - PubMed
-
- Assaf SY, Chung SH. Release of endogenous Zn2+ from brain tissue during activity. Nature. 1984;308:734–736. - PubMed
-
- Basolo F, Pearson RG. Mechanisms of inorganic reactions; a study of metal complexes in solution, Ed 2. Wiley; New York: 1967.
-
- Bers DM, Patton CW, Nuccitelli R. A practical guide to the preparation of Ca2+ buffers. Methods Cell Biol. 1994;40:3–29. - PubMed
-
- Bortolotto ZA, Clarke VR, Delany CM, Parry MC, Smolders I, Vignes M, Ho KH, Miu P, Brinton BT, Fantaske R, Ogden A, Gates M, Ornstein PL, Lodge D, Bleakman D, Collingridge GL. Kainate receptors are involved in synaptic plasticity. Nature. 1999;402:297–301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
