The neuronal form of adaptor protein-3 is required for synaptic vesicle formation from endosomes
- PMID: 11588176
- PMCID: PMC6763874
- DOI: 10.1523/JNEUROSCI.21-20-08034.2001
The neuronal form of adaptor protein-3 is required for synaptic vesicle formation from endosomes
Abstract
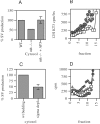
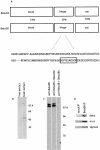
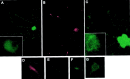
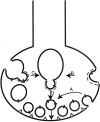
Heterotetrameric adaptor complexes vesiculate donor membranes. One of the adaptor protein complexes, AP-3, is present in two forms; one form is expressed in all tissues of the body, whereas the other is restricted to brain. Mice lacking both the ubiquitous and neuronal forms of AP-3 exhibit neurological disorders that are not observed in mice that are mutant only in the ubiquitous form. To begin to understand the role of neuronal AP-3 in neurological disease, we investigated its function in in vitro assays as well as its localization in neural tissue. In the presence of GTPgammaS both ubiquitous and neuronal forms of AP-3 can bind to purified synaptic vesicles. However, only the neuronal form of AP-3 can produce synaptic vesicles from endosomes in vitro. We also identified that the expression of neuronal AP-3 is limited to varicosities of neuronal-like processes and is expressed in most axons of the brain. Although the AP-2/clathrin pathway is the major route of vesicle production and the relatively minor neuronal AP-3 pathway is not necessary for viability, the absence of the latter could lead to the neurological abnormalities seen in mice lacking the expression of AP-3 in brain. In this study we have identified the first brain-specific function for a neuronal adaptor complex.
Figures




References
-
- Ball CL, Hunt SP, Robinson MS. Expression and localization of α-adaptin isoforms. J Cell Sci. 1995;108[Pt 8]:2865–2875. - PubMed
-
- Blagoveshchenskaya AD, Norcott JP, Cutler DF. Lysosomal targeting of P-selectin is mediated by a novel sequence within its cytoplasmic tail. J Biol Chem. 1998;273:2729–2737. - PubMed
-
- Clift-O'Grady L, Desnos C, Lichtenstein Y, Faundez V, Horng JT, Kelly RB. Reconstitution of synaptic vesicle biogenesis from PC12 cell membranes. Methods. 1998;16:150–159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
