Induction of alpha-synuclein aggregation by intracellular nitrative insult
- PMID: 11588178
- PMCID: PMC6763872
- DOI: 10.1523/JNEUROSCI.21-20-08053.2001
Induction of alpha-synuclein aggregation by intracellular nitrative insult
Abstract
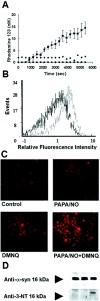
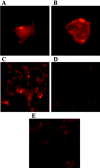
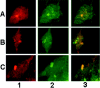
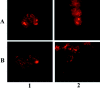
Brain lesions containing filamentous and aggregated alpha-synuclein are hallmarks of neurodegenerative synucleinopathies. Oxidative stress has been implicated in the formation of these lesions. Using HEK 293 cells stably transfected with wild-type and mutant alpha-synuclein, we demonstrated that intracellular generation of nitrating agents results in the formation of alpha-synuclein aggregates. Cells were exposed simultaneously to nitric oxide- and superoxide-generating compounds, and the intracellular formation of peroxynitrite was demonstrated by monitoring the oxidation of dihydrorhodamine 123 and the nitration of alpha-synuclein. Light microscopy using antibodies against alpha-synuclein and electron microscopy revealed the presence of perinuclear aggregates under conditions in which peroxynitrite was generated but not when cells were exposed to nitric oxide- or superoxide-generating compounds separately. alpha-Synuclein aggregates were observed in 20-30% of cells expressing wild-type or A53T mutant alpha-synuclein and in 5% of cells expressing A30P mutant alpha-synuclein. No evidence of synuclein aggregation was observed in untransfected cells or cells expressing beta-synuclein. In contrast, selective inhibition of the proteasome resulted in the formation of aggregates detected with antibodies to ubiquitin in the majority of the untransfected cells and cells expressing alpha-synuclein. However, alpha-synuclein did not colocalize with these aggregates, indicating that inhibition of the proteasome does not promote alpha-synuclein aggregation. In addition, proteasome inhibition did not alter the steady-state levels of alpha-synuclein, but addition of the lysosomotropic agent ammonium chloride significantly increased the amount of alpha-synuclein, indicating that lysosomes are involved in degradation of alpha-synuclein. Our data indicate that nitrative and oxidative insult may initiate pathogenesis of alpha-synuclein aggregates.
Figures





References
-
- Alvarez B, Ferrer-Sueta G, Freeman BA, Radi R. Kinetics of peroxynitrite reaction with amino acids and human serum albumin. J Biol Chem. 1999;274:842–848. - PubMed
-
- Ancolio K, Alves da Costa C, Ueda K, Checler F. α-Synuclein and the Parkinson's disease-related mutant Ala53Thr-α-synuclein do not undergo proteasomal degradation in HEK293 and neuronal cells. Neurosci Lett. 2000;285:79–82. - PubMed
-
- Bennett MC, Bishop JF, Leng Y, Chock PB, Chase TM, Mouradian MM. Degradation of α-synuclein by proteasome. J Biol Chem. 1999;274:33855–33858. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
