AMPA receptor channels with long-lasting desensitization in bipolar interneurons contribute to synaptic depression in a novel feedback circuit in layer 2/3 of rat neocortex
- PMID: 11588179
- PMCID: PMC6763867
- DOI: 10.1523/JNEUROSCI.21-20-08062.2001
AMPA receptor channels with long-lasting desensitization in bipolar interneurons contribute to synaptic depression in a novel feedback circuit in layer 2/3 of rat neocortex
Abstract
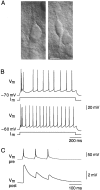
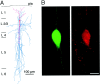
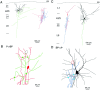
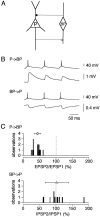
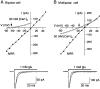
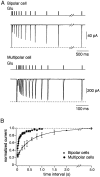
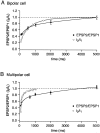
A novel, local inhibitory circuit in layer 2/3 of rat somatosensory cortex is described that connects pyramidal cells reciprocally with GABAergic vasoactive intestinal polypeptide-immunoreactive bipolar interneurons. In paired whole-cell recordings, the glutamatergic unitary responses (EPSPs or EPSCs) in bipolar cells evoked by repetitive (10 Hz) stimulation of a pyramidal cell show strong frequency-dependent depression. Unitary IPSPs evoked in pyramidal cells by repetitive stimulation of bipolar cells, on average, maintained their amplitude. This suggests that the excitatory synapses on bipolar cells act as a low-pass filter in the reciprocal pyramid-to-bipolar circuit. The EPSCs in bipolar cells are mediated predominantly by AMPA receptor (AMPAR) channels. AMPARs desensitize rapidly and recover slowly from desensitization evoked by a brief pulse of glutamate. In slices, reduction of AMPAR desensitization by cyclothiazide (50-100 microm) or conditioning steady-state desensitization induced by application of extracellular AMPA (50 nm) or glutamate (50 microm) strongly reduced synaptic depression. It is concluded that in the local circuits between pyramidal and bipolar cells the desensitization of AMPARs in bipolar cells contributes to low-pass feedback inhibition of layer 2/3 pyramidal neurons by bipolar cells.
Figures


References
-
- Bellingham MC, Walmsley B. A novel presynaptic inhibitory mechanism underlies paired pulse depression at a fast central synapse. Neuron. 1999;23:159–170. - PubMed
-
- Brusa R, Zimmerman F, Koh D-S, Feldmeyer D, Gass P, Seeburg P, Sprengel R. Early-onset epilepsy and postnatal lethality associated with an editing-deficient GluR-B allele in mice. Science. 1995;270:1677–1680. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
