Long-lasting aberrant tubulovesicular membrane inclusions accumulate in developing motoneurons after a sublethal excitotoxic insult: a possible model for neuronal pathology in neurodegenerative disease
- PMID: 11588180
- PMCID: PMC6763851
- DOI: 10.1523/JNEUROSCI.21-20-08072.2001
Long-lasting aberrant tubulovesicular membrane inclusions accumulate in developing motoneurons after a sublethal excitotoxic insult: a possible model for neuronal pathology in neurodegenerative disease
Abstract
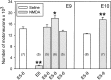
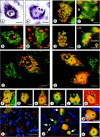
We have previously shown that chronic treatment of chick embryos [from embryonic day 5 (E5) to E9] with NMDA rescues spinal cord motoneurons (MNs) from programmed cell death. In this situation, MNs exhibit a reduced vulnerability to acute excitotoxic lesions and downregulate NMDA and AMPA-kainate receptors. Here, we report that this treatment results in long-lasting sublethal structural changes in MNs. In Nissl-stained sections from the spinal cord of NMDA-treated embryos, MNs display an area adjacent to an eccentrically positioned nucleus in which basophilia is excluded. Ultrastructurally, MNs accumulate tubulovesicular structures surrounded by Golgi stacks. Thiamine pyrophosphatase but not acid phosphatase was detected inside the tubulovesicular structures, which are resistant to disruption by brefeldin A or monensin. Immunocytochemistry reveals changes in the content and distribution of calcitonin gene-related peptide, the KDEL receptor, the early endosomal marker EEA1, and the recycling endosome marker Rab11, indicating that a dysfunction in membrane trafficking and protein sorting occurs in these MNs. FM1-43, a marker of the endocytic pathway, strongly accumulates in MNs from isolated spinal cords after chronic NMDA treatment. Changes in the distribution of cystatin C and presenilin-1 and an accumulation of amyloid precursor protein and beta-amyloid product were also observed in NMDA-treated MNs. None of these alterations involve an interruption of MN-target (muscle) connections, as detected by the retrograde tracing of MNs with cholera toxin B subunit. These results demonstrate that chronic NMDA treatment induces severe changes in the motoneuronal endomembrane system that may be related to some neuropathological alterations described in human MN disease.
Figures




Similar articles
-
Protein retention in the endoplasmic reticulum, blockade of programmed cell death and autophagy selectively occur in spinal cord motoneurons after glutamate receptor-mediated injury.Mol Cell Neurosci. 2005 Jun;29(2):283-98. doi: 10.1016/j.mcn.2005.03.003. Mol Cell Neurosci. 2005. PMID: 15911352
-
Excitotoxic motoneuron disease in chick embryo evolves with autophagic neurodegeneration and deregulation of neuromuscular innervation.J Neurosci Res. 2007 Sep;85(12):2726-40. doi: 10.1002/jnr.21174. J Neurosci Res. 2007. PMID: 17243177
-
Opposing effects of excitatory amino acids on chick embryo spinal cord motoneurons: excitotoxic degeneration or prevention of programmed cell death.J Neurosci. 1999 Dec 15;19(24):10803-12. doi: 10.1523/JNEUROSCI.19-24-10803.1999. J Neurosci. 1999. PMID: 10594063 Free PMC article.
-
Multiple targets for brefeldin A.Cell. 1991 Nov 1;67(3):449-51. doi: 10.1016/0092-8674(91)90517-3. Cell. 1991. PMID: 1934055 Review. No abstract available.
-
Functional symmetry of endomembranes.Mol Biol Cell. 2007 Apr;18(4):1430-6. doi: 10.1091/mbc.e06-10-0933. Epub 2007 Jan 31. Mol Biol Cell. 2007. PMID: 17267686 Free PMC article. Review.
Cited by
-
The Y172 Monoclonal Antibody Against p-c-Jun (Ser63) Is a Marker of the Postsynaptic Compartment of C-Type Cholinergic Afferent Synapses on Motoneurons.Front Cell Neurosci. 2020 Jan 24;13:582. doi: 10.3389/fncel.2019.00582. eCollection 2019. Front Cell Neurosci. 2020. PMID: 32038174 Free PMC article.
-
Motor neuron trophic factors: therapeutic use in ALS?Brain Res Rev. 2011 Jun 24;67(1-2):1-39. doi: 10.1016/j.brainresrev.2010.10.003. Epub 2010 Oct 21. Brain Res Rev. 2011. PMID: 20971133 Free PMC article. Review.
-
Insulin-like growth factor-I for the treatment of amyotrophic lateral sclerosis.Amyotroph Lateral Scler. 2009 Apr;10(2):63-73. doi: 10.1080/17482960802160370. Amyotroph Lateral Scler. 2009. PMID: 18608100 Free PMC article. Review.
-
Neural cell adhesion molecule promotes accumulation of TGN organelles at sites of neuron-to-neuron contacts.J Cell Biol. 2002 Nov 25;159(4):649-61. doi: 10.1083/jcb.200205098. Epub 2002 Nov 18. J Cell Biol. 2002. PMID: 12438412 Free PMC article.
References
-
- Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. - PubMed
-
- Barron KD, Chiang TY, Daniels AC, Doolin PF. Subcellular accompaniments of axon reaction in cervical motoneurons of the cat. Prog Neuropathol. 1971;1:255–280.
-
- Calderó J, Casanovas A, Sorribas A, Esquerda JE. Calcitonin gene-related peptide in rat spinal cord motoneurons: subcellular distribution and changes induced by axotomy. Neuroscience. 1992;48:449–461. - PubMed
-
- Calderó J, Ciutat D, Lladó J, Castán E, Oppenheim RW, Esquerda JE. Effects of excitatory amino acids on neuromuscular development in chick embryo. J Comp Neurol. 1997;387:73–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
