Rna-binding protein Musashi2: developmentally regulated expression in neural precursor cells and subpopulations of neurons in mammalian CNS
- PMID: 11588182
- PMCID: PMC6763847
- DOI: 10.1523/JNEUROSCI.21-20-08091.2001
Rna-binding protein Musashi2: developmentally regulated expression in neural precursor cells and subpopulations of neurons in mammalian CNS
Abstract
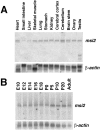
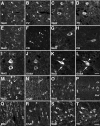
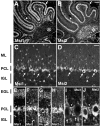
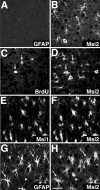
Musashi1 (Msi1) is a mammalian neural RNA-binding protein highly enriched in neural precursor cells that are capable of generating both neurons and glia during embryonic and postnatal CNS development. Here, we identified Musashi2 (Msi2), a novel mammalian RNA-binding protein that exhibits high sequence similarity to Msi1. The Msi2 transcript appeared to be distributed ubiquitously in a wide variety of tissues, consistent with the mRNA distribution of its Xenopus homolog, xrp1. However, the present study revealed cell type-specific and developmentally regulated expression of Msi2 in the mammalian CNS. Interestingly, Msi2 was expressed prominently in precursor cells in the ventricular zone and subventricular zone with the same pattern as Msi1 throughout CNS development. In the postnatal and adult CNS, this concurrent expression of Msi2 and Msi1 was seen in cells of the astrocyte lineage, including ependymal cells, a possible source for postnatal CNS stem cells. During neurogenesis, the expression of both Msi2 and Msi1 was lost in most postmitotic neurons, whereas Msi2 expression persisted in a subset of neuronal lineage cells, such as parvalbumin-containing GABA neurons in the neocortex and neurons in several nuclei of the basal ganglia. Msi2 may have a unique role that is required for the generation and/or maintenance of specific neuronal lineages. Furthermore, in vitro studies showed that Msi2 and Msi1 have similar RNA-binding specificity. These two RNA-binding proteins may exert common functions in neural precursor cells by regulating gene expression at the post-transcriptional level.
Figures







References
-
- Akamatsu W, Okano HJ, Osumi N, Inoue T, Nakamura S, Sakakibara S, Miura M, Matsuo N, Darnell RB, Okano H. Mammalian ELAV-like neuronal RNA-binding proteins HuB and HuC promote neuronal development in both the central and peripheral nervous systems. Proc Natl Acad Sci USA. 1999;96:9885–9890. - PMC - PubMed
-
- Altman J. Postnatal development of the cerebellar cortex in the rat. J Comp Neurol. 1972;145:353–398. - PubMed
-
- Amano T, Hamprecht B, Kemper W. High activity of choline acetyltransferase induced in neuroblastoma × glioma hybrid cells. Exp Cell Res. 1974;85:399–408. - PubMed
-
- Barami K, Iversen K, Furneaux H, Goldman SA. Early expression of Hu proteins by newly generated neurons of the adult avian forebrain. J Neurobiol. 1995;28:82–101. - PubMed
-
- Bayer SA, Altman J. Neocortical development. Raven; New York: 1991.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
