Neuroprotection through delivery of glial cell line-derived neurotrophic factor by neural stem cells in a mouse model of Parkinson's disease
- PMID: 11588183
- PMCID: PMC6763865
- DOI: 10.1523/JNEUROSCI.21-20-08108.2001
Neuroprotection through delivery of glial cell line-derived neurotrophic factor by neural stem cells in a mouse model of Parkinson's disease
Abstract
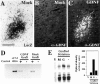
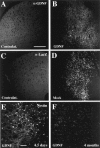
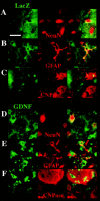
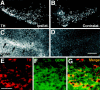
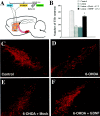
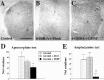
Neural stem cells (NSCs) have been proposed as tools for treating neurodegeneration because of their capacity to give rise to cell types appropriate to the structure in which they are grafted. In the present work, we explore the ability of NSCs to stably express transgenes and locally deliver soluble molecules with neuroprotective activity, such as glial cell line-derived neurotrophic factor (GDNF). NSCs engineered to release GDNF engrafted well in the host striatum, integrated and gave rise to neurons, astrocytes, and oligodendrocytes, and maintained stable high levels of GDNF expression for at least 4 months. The therapeutic potential of intrastriatal GDNF-NSCs grafts was tested in a mouse 6-hydroxydopamine model of Parkinson's disease. We found that GDNF-NSCs prevented the degeneration of dopaminergic neurons in the substantia nigra and reduced behavioral impairment in these animals. Thus, our results demonstrate that NSCs efficiently express therapeutic levels of GDNF in vivo, suggesting a use for NSCs engineered to release neuroprotective molecules in the treatment of neurodegenerative disorders, including Parkinson's disease.
Figures


Similar articles
-
Astrocyte delivery of glial cell line-derived neurotrophic factor in a mouse model of Parkinson's disease.Exp Neurol. 2002 Apr;174(2):230-42. doi: 10.1006/exnr.2002.7877. Exp Neurol. 2002. PMID: 11922664
-
Lentiviral vectors as a gene delivery system in the mouse midbrain: cellular and behavioral improvements in a 6-OHDA model of Parkinson's disease using GDNF.Exp Neurol. 2000 Jul;164(1):15-24. doi: 10.1006/exnr.2000.7409. Exp Neurol. 2000. PMID: 10877911
-
Towards a neuroprotective gene therapy for Parkinson's disease: use of adenovirus, AAV and lentivirus vectors for gene transfer of GDNF to the nigrostriatal system in the rat Parkinson model.Brain Res. 2000 Dec 15;886(1-2):82-98. doi: 10.1016/s0006-8993(00)02915-2. Brain Res. 2000. PMID: 11119690 Review.
-
Additive effect of glial cell line-derived neurotrophic factor and neurotrophin-4/5 on rat fetal nigral explant cultures.Neuroscience. 2001;108(2):273-84. doi: 10.1016/s0306-4522(01)00418-3. Neuroscience. 2001. PMID: 11734360
-
Glial cell line-derived neurotrophic factor (GDNF): a drug candidate for the treatment of Parkinson's disease.J Neurol. 1998 Nov;245(11 Suppl 3):P35-42. doi: 10.1007/pl00007744. J Neurol. 1998. PMID: 9808338 Review.
Cited by
-
Carbon nanotubes impregnated with subventricular zone neural progenitor cells promotes recovery from stroke.Int J Nanomedicine. 2012;7:2751-65. doi: 10.2147/IJN.S30273. Epub 2012 Jun 1. Int J Nanomedicine. 2012. PMID: 22701320 Free PMC article.
-
Stem cell and precursor cell therapy.Neuromolecular Med. 2002;2(3):233-49. doi: 10.1385/NMM:2:3:233. Neuromolecular Med. 2002. PMID: 12622402 Review.
-
Optimization of Tet1 ligand density in HPMA-co-oligolysine copolymers for targeted neuronal gene delivery.Biomaterials. 2013 Dec;34(37):9632-7. doi: 10.1016/j.biomaterials.2013.08.045. Epub 2013 Sep 13. Biomaterials. 2013. PMID: 24041424 Free PMC article.
-
Apoptosis in glioma-bearing rats after neural stem cell transplantation.Neural Regen Res. 2013 Jul 5;8(19):1793-802. doi: 10.3969/j.issn.1673-5374.2013.19.007. Neural Regen Res. 2013. PMID: 25206476 Free PMC article.
-
Pharmacokinetics and bioactivity of glial cell line-derived factor (GDNF) and neurturin (NTN) infused into the rat brain.Neuropharmacology. 2010 Jun;58(7):1114-21. doi: 10.1016/j.neuropharm.2010.02.002. Epub 2010 Feb 11. Neuropharmacology. 2010. PMID: 20153340 Free PMC article.
References
-
- Åkerud P, Alberch J, Eketjäll S, Wagner J, Arenas E. Differential effects of GDNF and Neurturin on developing and adult substantia nigra dopaminergic neurons. J Neurochem. 1999;73:70–78. - PubMed
-
- Arenas E, Trupp M, Åkerud P, Ibáñez CF. GDNF prevents degeneration and promotes the phenotype of brain noradrenergic neurons in vivo. Neuron. 1995;15:1465–1473. - PubMed
-
- Barneoud P, Parmentier S, Mazadier J, Miquet JM, Boireau A, Dubedat P, Blanchard J-C. Effects of complete and partial lesions of the dopaminergic mesotelencephalic system on skilled forelimb use in the rat. Neuroscience. 1995;67:837–848. - PubMed
-
- Beck K, Valverde J, Alexi T, Poulsen K, Moffat B, Vandlen R, Rosenthal A, Hefti F. Mesencephalic dopaminergic neurons protected by GDNF from axotomy-induced degeneration in the adult brain. Nature. 1995;373:339–341. - PubMed
-
- Benedetti S, Pirola B, Pollo B, Magrassi L, Bruzzone MG, Rigamonti D, Galli R, Selleri S, Di Meco F, De Fraja C, Vescovi A, Cattaneo E, Finocchiaro G. Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med. 2000;6:447–450. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
