Deposition of the NG2 proteoglycan at nodes of Ranvier in the peripheral nervous system
- PMID: 11588184
- PMCID: PMC6763877
- DOI: 10.1523/JNEUROSCI.21-20-08119.2001
Deposition of the NG2 proteoglycan at nodes of Ranvier in the peripheral nervous system
Abstract
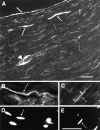
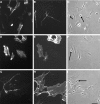
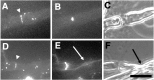
The node of Ranvier is a complex macromolecular assembly of ion channels and other proteins that is specialized for the rapid propagation of the action potential. A full understanding of the processes responsible for the assembly and maintenance of the node requires first the identification and characterization of the proteins found there. Here we show that NG2, a structurally unique chondroitin sulfate proteoglycan, is a molecular component of the node of Ranvier in the peripheral nervous system. In adult sciatic nerve, NG2 is (1) associated with thin, elongated fibroblast-like cells, (2) on some but not all basal laminae, and (3) at nodes of Ranvier. At the nodes, NG2 is restricted to the nodal gap and is absent from the paranodal or juxtaparanodal region. In dissociated cell cultures of adult sciatic nerve, perineurial fibroblasts but not Schwann cells express NG2 on their surfaces. Approximately 45% of the total NG2 in peripheral nerves is in a soluble, rather than particulate, subcellular compartment. NG2 is also present in membrane fractions that also contain high levels of voltage-dependent sodium channels, caspr, and neuron-glia related cell adhesion molecule. These medium-density membranes likely correspond to the nodal and paranodal region of the axon-Schwann cell unit. These results suggest a model in which perineurial fibroblasts secrete or shed NG2, which subsequently associates with nodes of Ranvier. The growth-inhibitory and anti-adhesive properties of NG2 may limit the lateral extension of myelinating Schwann cells as nodes mature. NG2 may also participate in the barrier functions of the perineurial linings of the nerve.
Figures




Similar articles
-
Heterogeneity of astrocyte and NG2 cell insertion at the node of ranvier.J Comp Neurol. 2017 Feb 15;525(3):535-552. doi: 10.1002/cne.24083. Epub 2016 Aug 18. J Comp Neurol. 2017. PMID: 27448245 Free PMC article.
-
Gliomedin mediates Schwann cell-axon interaction and the molecular assembly of the nodes of Ranvier.Neuron. 2005 Jul 21;47(2):215-29. doi: 10.1016/j.neuron.2005.06.026. Neuron. 2005. PMID: 16039564
-
Differential expression of proteoglycans at central and peripheral nodes of Ranvier.Glia. 2005 Dec;52(4):301-8. doi: 10.1002/glia.20245. Glia. 2005. PMID: 16035076
-
The Nodes of Ranvier: Molecular Assembly and Maintenance.Cold Spring Harb Perspect Biol. 2015 Sep 9;8(3):a020495. doi: 10.1101/cshperspect.a020495. Cold Spring Harb Perspect Biol. 2015. PMID: 26354894 Free PMC article. Review.
-
[New insights on the organization of the nodes of Ranvier].Rev Neurol (Paris). 2014 Dec;170(12):819-24. doi: 10.1016/j.neurol.2014.03.017. Epub 2014 Nov 20. Rev Neurol (Paris). 2014. PMID: 25459119 Review. French.
Cited by
-
Neutralization of inhibitory molecule NG2 improves synaptic transmission, retrograde transport, and locomotor function after spinal cord injury in adult rats.J Neurosci. 2013 Feb 27;33(9):4032-43. doi: 10.1523/JNEUROSCI.4702-12.2013. J Neurosci. 2013. PMID: 23447612 Free PMC article.
-
The reactions and role of NG2 glia in spinal cord injury.Brain Res. 2016 May 1;1638(Pt B):199-208. doi: 10.1016/j.brainres.2015.07.026. Epub 2015 Jul 29. Brain Res. 2016. PMID: 26232070 Free PMC article. Review.
-
Oligodendrocytes regulate formation of nodes of Ranvier via the recognition molecule OMgp.Neuron Glia Biol. 2006 Aug;2(3):151-64. doi: 10.1017/S1740925X06000251. Neuron Glia Biol. 2006. PMID: 17364021 Free PMC article.
-
Heterogeneity of astrocyte and NG2 cell insertion at the node of ranvier.J Comp Neurol. 2017 Feb 15;525(3):535-552. doi: 10.1002/cne.24083. Epub 2016 Aug 18. J Comp Neurol. 2017. PMID: 27448245 Free PMC article.
-
Chondroitinase ABC reduces time to muscle reinnervation and improves functional recovery after sciatic nerve transection in rats.J Neurophysiol. 2012 Feb;107(3):747-57. doi: 10.1152/jn.00887.2011. Epub 2011 Nov 2. J Neurophysiol. 2012. PMID: 22049333 Free PMC article.
References
-
- Arroyo EJ, Scherer SS. On the molecular architecture of myelinated fibers. Histochem Cell Biol. 2000;113:1–18. - PubMed
-
- Bellen HJ, Lu Y, Beckstead R, Bhat MA. Neurexin IV, caspr and paranodin—novel members of the neurexin family: encounters of axons and glia. Trends Neurosci. 1998;21:444–449. - PubMed
-
- Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. - PubMed
-
- Bothwell M. Tissue localization of nerve growth factor and nerve growth factor receptors. Curr Top Microbiol Immunol. 1991;165:55–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
