Decreased glutamate receptor 2 expression and enhanced epileptogenesis in immature rat hippocampus after perinatal hypoxia-induced seizures
- PMID: 11588188
- PMCID: PMC6763879
- DOI: 10.1523/JNEUROSCI.21-20-08154.2001
Decreased glutamate receptor 2 expression and enhanced epileptogenesis in immature rat hippocampus after perinatal hypoxia-induced seizures
Abstract
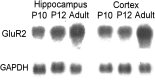
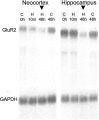
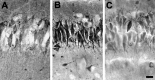
Hypoxic encephalopathy is the most common cause of neonatal seizures and can lead to chronic epilepsy. In rats at postnatal days 10-12 (P10-12), global hypoxia induces spontaneous seizures and chronically decreases seizure threshold, thus mimicking clinical aspects of neonatal hypoxia. We have shown previously that the acute and chronic epileptogenic effects of hypoxia are age-dependent and require AMPA receptor activation. In this study, we aimed to determine whether hypoxia-induced seizures and epileptogenesis are associated with maturational and seizure-induced changes in AMPA receptor composition and function. Northern and Western blots indicated that glutamate receptor 2 (GluR2) mRNA and protein expression were significantly lower in neocortex and hippocampus at P10-12 compared with adult. After hypoxia-induced seizures at P10, GluR2 mRNA was significantly decreased within 48 hr, and GluR2 protein was significantly decreased within 96 hr. AMPA-induced Co(2+) uptake by neurons in hippocampal slices indicated higher expression of Ca(2+)-permeable AMPA receptors in immature pyramidal neurons compared with adult. In slices obtained 96 hr after hypoxia-induced seizures, AMPA-induced Co(2+) uptake was significantly increased compared with age-matched controls, and field recordings revealed increased tetanus-induced afterdischarges that could be kindled in the absence of NMDA receptor activation. In situ end labeling showed no acute or delayed cell death after hypoxia-induced seizures. Our results indicate that susceptibility to hypoxia-induced seizures occurs during a developmental stage in which the expression of Ca(2+)-permeable AMPA receptors is relatively high. Furthermore, perinatal hypoxia-induced seizures induce increased expression of Ca(2+)-permeable AMPA receptors and an increased capacity for AMPA receptor-mediated epileptogenesis without inducing cell death.
Figures







Similar articles
-
Decreased IH in hippocampal area CA1 pyramidal neurons after perinatal seizure-inducing hypoxia.Epilepsia. 2006 Jun;47(6):1023-8. doi: 10.1111/j.1528-1167.2006.00574.x. Epilepsia. 2006. PMID: 16822248
-
Acute and chronic increases in excitability in rat hippocampal slices after perinatal hypoxia In vivo.J Neurophysiol. 1998 Jan;79(1):73-81. doi: 10.1152/jn.1998.79.1.73. J Neurophysiol. 1998. PMID: 9425178
-
NBQX or topiramate treatment after perinatal hypoxia-induced seizures prevents later increases in seizure-induced neuronal injury.Epilepsia. 2004 Jun;45(6):569-75. doi: 10.1111/j.0013-9580.2004.69103.x. Epilepsia. 2004. PMID: 15144420
-
The role of glutamate receptor maturation in perinatal seizures and brain injury.Int J Dev Neurosci. 2002 Jun-Aug;20(3-5):339-47. doi: 10.1016/s0736-5748(02)00012-6. Int J Dev Neurosci. 2002. PMID: 12175872 Review.
-
The AMPAR subunit GluR2: still front and center-stage.Brain Res. 2000 Dec 15;886(1-2):190-207. doi: 10.1016/s0006-8993(00)02951-6. Brain Res. 2000. PMID: 11119696 Review.
Cited by
-
Prion-like mechanisms in epileptogenesis.Neurol Sci. 2013 Jun;34(6):1035-8. doi: 10.1007/s10072-012-1148-0. Epub 2012 Jul 10. Neurol Sci. 2013. PMID: 22777569
-
Epilepsy and epileptic syndrome.Adv Exp Med Biol. 2012;724:99-113. doi: 10.1007/978-1-4614-0653-2_8. Adv Exp Med Biol. 2012. PMID: 22411237 Free PMC article. Review.
-
A systematic review comparing neurodevelopmental outcome in term infants with hypoxic and vascular brain injury with and without seizures.BMC Pediatr. 2018 May 2;18(1):147. doi: 10.1186/s12887-018-1116-9. BMC Pediatr. 2018. PMID: 29720158 Free PMC article.
-
Epileptogenesis in the developing brain: what can we learn from animal models?Epilepsia. 2007;48 Suppl 5(Suppl 5):2-6. doi: 10.1111/j.1528-1167.2007.01281.x. Epilepsia. 2007. PMID: 17910574 Free PMC article. Review.
-
Dorsal and ventral hippocampus modulate autonomic responses but not behavioral consequences associated to acute restraint stress in rats.PLoS One. 2013 Oct 17;8(10):e77750. doi: 10.1371/journal.pone.0077750. eCollection 2013. PLoS One. 2013. PMID: 24147071 Free PMC article.
References
-
- Bochet P, Audinat E, Lambolez B, Crepel F, Rossier J, Iino M, Tsuzuki K, Ozawa S. Subunit composition at the single-cell level explains functional properties of a glutamate-gated channel. Neuron. 1994;12:383–388. - PubMed
-
- Burnashev N, Monyer H, Seeburg PH, Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992;8:189–198. - PubMed
-
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. - PubMed
-
- Durand GM, Zukin RS. Developmental regulation of RNAs encoding rat brain kainate/AMPA receptors: a Northern analysis study. J Neurochem. 1993;61:2239–2246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
