Selective immunolesions of cholinergic neurons in mice: effects on neuroanatomy, neurochemistry, and behavior
- PMID: 11588189
- PMCID: PMC6763842
- DOI: 10.1523/JNEUROSCI.21-20-08164.2001
Selective immunolesions of cholinergic neurons in mice: effects on neuroanatomy, neurochemistry, and behavior
Abstract
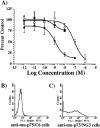
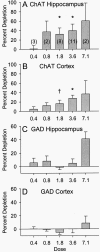
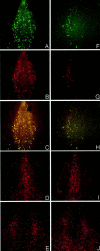
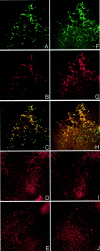
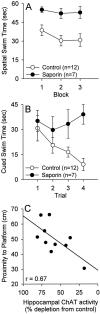
The ability to selectively lesion mouse basal forebrain cholinergic neurons would permit experimental examination of interactions between cholinergic functional loss and genetic factors associated with neurodegenerative disease. We developed a selective toxin for mouse basal forebrain cholinergic neurons by conjugating saporin (SAP), a ribosome-inactivating protein, to a rat monoclonal antibody against the mouse p75 nerve growth factor (NGF) receptor (anti-murine-p75). The toxin proved effective and selective in vitro and in vivo. Intracerebroventricular injections of anti-murine-p75-SAP produced a dose-dependent loss of choline acetyltransferase (ChAT) activity in the hippocampus and neocortex without affecting glutamic acid decarboxylase (GAD) activity. Hippocampal ChAT depletions induced by the immunotoxin were consistently greater than neocortical depletions. Immunohistochemical analysis revealed a dose-dependent loss of cholinergic neurons in the medial septum (MS) but no marked loss of cholinergic neurons in the nucleus basalis magnocellularis after intracerebroventricular injection of the toxin. No loss of noncholinergic neurons in the MS was apparent, nor could we detect loss of noncholinergic cerebellar Purkinje cells, which also express p75. Behavioral analysis suggested a spatial learning deficit in anti-murine-p75-SAP-lesioned mice, based on a correlation between a loss of hippocampal ChAT activity and impairment in Morris water maze performance. Our results indicate that we have developed a specific cholinergic immunotoxin for mice. They also suggest possible functional differences in the mouse and rat cholinergic systems, which may be of particular significance in attempts to develop animal models of human diseases, such as Alzheimer's disease, which are associated with impaired cholinergic function.
Figures


References
-
- Arters J, Hohmann CF, Mills J, Olaghere O, Berger-Sweeney J. Sexually dimorphic responses to neonatal basal forebrain lesions in mice. I. Behavior and neurochemistry. J Neurobiol. 1998;37:582–594. - PubMed
-
- Baxter MG, Chiba AA. Cognitive functions of the basal forebrain. Curr Opin Neurobiol. 1999;9:178–183. - PubMed
-
- Berger-Sweeney J, Berger UV, Sharma M, Paul CA. Effects of carbon dioxide-induced anesthesia on cholinergic parameters in rat brain. Lab Anim Sci. 1994a;44:369–371. - PubMed
-
- Bornemann K, Staufenbiel M. Transgenic mouse models of Alzheimer's disease. Ann NY Acad Sci. 2000;908:260–266. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
