5-hydroxytryptamine (5-HT)1A autoreceptor adaptive changes in substance P (neurokinin 1) receptor knock-out mice mimic antidepressant-induced desensitization
- PMID: 11588191
- PMCID: PMC6763873
- DOI: 10.1523/JNEUROSCI.21-20-08188.2001
5-hydroxytryptamine (5-HT)1A autoreceptor adaptive changes in substance P (neurokinin 1) receptor knock-out mice mimic antidepressant-induced desensitization
Abstract
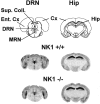
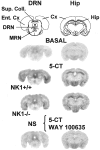
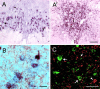
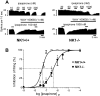
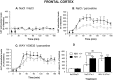
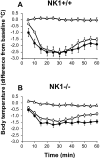
Antagonists at substance P receptors of the neurokinin 1 (NK1) type have been shown to represent a novel class of antidepressant drugs, with comparable clinical efficacy to the selective serotonin (5-HT) reuptake inhibitors (SSRIs). Because 5-HT(1A) receptors may be critically involved in the mechanisms of action of SSRIs, we examined whether these receptors could also be affected in a model of whole-life blockade of NK1 receptors, i.e. knock-out mice lacking the latter receptors (NK1-/-). 5-HT(1A) receptor labeling by the selective antagonist radioligand [(3)H]N-[2-[4-(2-methoxyphenyl)1-piperazinyl]-ethyl]-N-(2-pyridinyl)-cyclohexanecarboxamide (WAY 100635) and 5-HT(1A)-dependent [(35)S]GTP-gamma-S binding at the level of the dorsal raphe nucleus (DRN) in brain sections, as well as the concentration of 5-HT(1A) mRNA in the anterior raphe area were significantly reduced (-19 to -46%) in NK1-/- compared with NK1+/+ mice. Furthermore, a approximately 10-fold decrease in the potency of the 5-HT(1A) receptor agonist ipsapirone to inhibit the discharge of serotoninergic neurons in the dorsal raphe nucleus within brainstem slices, and reduced hypothermic response to 8-OH-DPAT, were noted in NK1-/- versus NK1+/+ mice. On the other hand, cortical 5-HT overflow caused by systemic injection of the SSRI paroxetine was four- to sixfold higher in freely moving NK1-/- mutants than in wild-type NK1+/+ mice. Accordingly, the constitutive lack of NK1 receptors appears to be associated with a downregulation/functional desensitization of 5-HT(1A) autoreceptors resembling that induced by chronic treatment with SSRI antidepressants. Double immunocytochemical labeling experiments suggest that such a heteroregulation of 5-HT(1A) autoreceptors in NK1-/- mutants does not reflect the existence of direct NK1-5-HT(1A) receptor interactions in normal mice.
Figures
Similar articles
-
Sustained pharmacological blockade of NK1 substance P receptors causes functional desensitization of dorsal raphe 5-HT 1A autoreceptors in mice.J Neurochem. 2005 Dec;95(6):1713-23. doi: 10.1111/j.1471-4159.2005.03488.x. Epub 2005 Oct 7. J Neurochem. 2005. PMID: 16219031
-
Functional consequences of 5-HT transporter gene disruption on 5-HT(1a) receptor-mediated regulation of dorsal raphe and hippocampal cell activity.J Neurosci. 2001 Mar 15;21(6):2178-85. doi: 10.1523/JNEUROSCI.21-06-02178.2001. J Neurosci. 2001. PMID: 11245702 Free PMC article.
-
Agonist-induced internalization of serotonin-1a receptors in the dorsal raphe nucleus (autoreceptors) but not hippocampus (heteroreceptors).J Neurosci. 2001 Nov 1;21(21):8378-86. doi: 10.1523/JNEUROSCI.21-21-08378.2001. J Neurosci. 2001. PMID: 11606626 Free PMC article.
-
Control of dorsal raphé 5-HT function by multiple 5-HT(1) autoreceptors: parallel purposes or pointless plurality?Trends Neurosci. 2000 Oct;23(10):459-65. doi: 10.1016/s0166-2236(00)01631-3. Trends Neurosci. 2000. PMID: 11006462 Review.
-
Microdialysis approach to study serotonin outflow in mice following selective serotonin reuptake inhibitors and substance P (neurokinin 1) receptor antagonist administration: a review.Curr Drug Targets. 2006 Feb;7(2):187-201. doi: 10.2174/138945006775515428. Curr Drug Targets. 2006. PMID: 16475960 Review.
Cited by
-
Innovative approaches for the development of antidepressant drugs: current and future strategies.NeuroRx. 2005 Oct;2(4):590-611. doi: 10.1602/neurorx.2.4.590. NeuroRx. 2005. PMID: 16489368 Free PMC article. Review.
-
Validation of the forced swim test in Drosophila, and its use to demonstrate psilocybin has long-lasting antidepressant-like effects in flies.Sci Rep. 2022 Jun 15;12(1):10019. doi: 10.1038/s41598-022-14165-2. Sci Rep. 2022. PMID: 35705666 Free PMC article.
-
The future of antidepressant pharmacotherapy.World Psychiatry. 2003 Feb;2(1):3-8. World Psychiatry. 2003. PMID: 16946878 Free PMC article. No abstract available.
-
Glutamatergic drive of the dorsal raphe nucleus.J Chem Neuroanat. 2011 Jul;41(4):247-55. doi: 10.1016/j.jchemneu.2011.04.004. Epub 2011 Apr 27. J Chem Neuroanat. 2011. PMID: 21550397 Free PMC article. Review.
-
VGLUT3 (vesicular glutamate transporter type 3) contribution to the regulation of serotonergic transmission and anxiety.J Neurosci. 2010 Feb 10;30(6):2198-210. doi: 10.1523/JNEUROSCI.5196-09.2010. J Neurosci. 2010. PMID: 20147547 Free PMC article.
References
-
- Albert PR, Zhou QY, Van Tol HH, Bunzow JR, Civelli O. Cloning, functional expression and mRNA tissue distribution of the rat 5-HT1A receptor gene. J Biol Chem. 1990;265:5825–5832. - PubMed
-
- Artigas F, Romero L, De Montigny C, Blier P. Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends Neurosci. 1996;19:378–383. - PubMed
-
- Asberg M, Thoren P, Träksman L. Serotonin depression in a biochemical subgroup within the affective disorders. Life Sci. 1976;191:478–480. - PubMed
-
- Barker R. Substance P and neurodegenerative disorders. A speculative review. Neuropeptides. 1991;20:73–78. - PubMed
-
- Bel N, Artigas F. Chronic treatment with fluvoxamine increases extracellular serotonin in frontal cortex but not in raphe nuclei. Synapse. 1993;15:243–245. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
