Reduction in opioid- and cannabinoid-induced antinociception in rhesus monkeys after bilateral lesions of the amygdaloid complex
- PMID: 11588195
- PMCID: PMC6763858
- DOI: 10.1523/JNEUROSCI.21-20-08238.2001
Reduction in opioid- and cannabinoid-induced antinociception in rhesus monkeys after bilateral lesions of the amygdaloid complex
Abstract
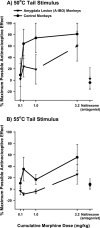
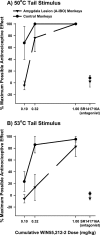
The amygdaloid complex is a prominent temporal lobe region that is associated with "emotional" information processing. Studies in the rodent have also recently implicated the amygdala in the processing and modulation of pain sensation, the experience of which involves a considerable emotional component in humans. In the present study, we sought to establish the relevance of the amygdala to pain modulation in humans by investigating the contribution of this region to antinociceptive processes in nonhuman primates. Using magnetic resonance imaging guidance, the amygdaloid complex was lesioned bilaterally in six rhesus monkeys (Macaca mulatta) through microinjection of the neurotoxin ibotenic acid. This procedure resulted in substantial neuronal cell loss in all nuclear subdivisions of this structure. In awake unoperated control monkeys, systemic administration of the prototypical opioid morphine or the cannabinoid receptor agonist WIN55,212-2 produced dose-dependent antinociception on a warm-water tail-withdrawal assay. The antinociceptive effects of each drug were reversible with an appropriate antagonist. In monkeys with bilateral amygdala lesions, however, the antinociceptive effects of each drug were significantly reduced. These results constitute the first causal data demonstrating the necessity of neurons in a specific brain region for the full expression of opioid- and cannabinoid-induced antinociception in the primate. Because our amygdala-lesioned monkeys exhibited both a reduction in antinociception and a reduction in behavioral indices of fear (Emery et al., 2001), the possibility should be considered that, in the primate, "antinociceptive circuitry" and "fear circuitry" overlap at the level of the amygdala.
Figures
Similar articles
-
Interactions between μ-opioid receptor agonists and cannabinoid receptor agonists in rhesus monkeys: antinociception, drug discrimination, and drug self-administration.J Pharmacol Exp Ther. 2013 Jun;345(3):354-62. doi: 10.1124/jpet.113.204099. Epub 2013 Mar 27. J Pharmacol Exp Ther. 2013. PMID: 23536317 Free PMC article.
-
The rodent amygdala contributes to the production of cannabinoid-induced antinociception.Neuroscience. 2003;120(4):1157-70. doi: 10.1016/s0306-4522(03)00356-7. Neuroscience. 2003. PMID: 12927220
-
Combined Treatment with Morphine and Δ9-Tetrahydrocannabinol in Rhesus Monkeys: Antinociceptive Tolerance and Withdrawal.J Pharmacol Exp Ther. 2016 May;357(2):357-66. doi: 10.1124/jpet.115.231381. Epub 2016 Mar 2. J Pharmacol Exp Ther. 2016. PMID: 26937020 Free PMC article.
-
Participation of the opioid system in cannabinoid-induced antinociception and emotional-like responses.Eur Neuropsychopharmacol. 2003 Dec;13(6):401-10. doi: 10.1016/j.euroneuro.2003.08.001. Eur Neuropsychopharmacol. 2003. PMID: 14636956 Review.
-
Toward a holistic view of value and social processing in the amygdala: Insights from primate behavioral neurophysiology.Behav Brain Res. 2021 Aug 6;411:113356. doi: 10.1016/j.bbr.2021.113356. Epub 2021 May 11. Behav Brain Res. 2021. PMID: 33989727 Free PMC article. Review.
Cited by
-
Cannabinoid Receptors and Their Relationship With Chronic Pain: A Narrative Review.Cureus. 2020 Sep 14;12(9):e10436. doi: 10.7759/cureus.10436. Cureus. 2020. PMID: 33072446 Free PMC article. Review.
-
Synaptic plasticity in the amygdala in a model of arthritic pain: differential roles of metabotropic glutamate receptors 1 and 5.J Neurosci. 2003 Jan 1;23(1):52-63. doi: 10.1523/JNEUROSCI.23-01-00052.2003. J Neurosci. 2003. PMID: 12514201 Free PMC article.
-
Interactions between μ-opioid receptor agonists and cannabinoid receptor agonists in rhesus monkeys: antinociception, drug discrimination, and drug self-administration.J Pharmacol Exp Ther. 2013 Jun;345(3):354-62. doi: 10.1124/jpet.113.204099. Epub 2013 Mar 27. J Pharmacol Exp Ther. 2013. PMID: 23536317 Free PMC article.
-
Tuberoinfundibular peptide of 39 residues (TIP39) signaling modulates acute and tonic nociception.Exp Neurol. 2010 Nov;226(1):68-83. doi: 10.1016/j.expneurol.2010.08.004. Epub 2010 Aug 6. Exp Neurol. 2010. PMID: 20696160 Free PMC article.
-
[Endogenous cannabinoid system. Effect on neuronal plasticity and pain memory].Schmerz. 2005 Nov;19(6):521-7. doi: 10.1007/s00482-004-0342-2. Schmerz. 2005. PMID: 16328555 German.
References
-
- Adler LJ, Gyulai FE, Diehl DJ, Mintun MA, Winter PM, Firestone LL. Regional brain activity changes associated with fentanyl analgesia elucidated by positron emission tomography. Anesth Analg 84 1997. 120 126 [Erratum (1997) 84:949] - PubMed
-
- Aggleton JP. The contribution of the amygdala to normal and abnormal emotional states. Trends Neurosci. 1993;16:328–333. - PubMed
-
- Alvarez-Royo P, Clower RP, Zola-Morgan S, Squire LR. Stereotaxic lesions of the hippocampus in monkeys: determination of surgical coordinates and analysis of lesions using magnetic resonance imaging. J Neurosci Methods. 1991;38:223–232. - PubMed
-
- Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis). J Comp Neurol. 1984;230:465–496. - PubMed
-
- Amaral DG, Price JL, Pitkanen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. Wiley; New York: 1992. pp. 1–66.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
