The enterins: a novel family of neuropeptides isolated from the enteric nervous system and CNS of Aplysia
- PMID: 11588196
- PMCID: PMC6763844
- DOI: 10.1523/JNEUROSCI.21-20-08247.2001
The enterins: a novel family of neuropeptides isolated from the enteric nervous system and CNS of Aplysia
Abstract
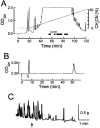
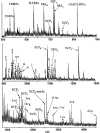
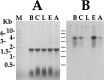
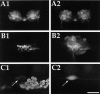
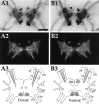
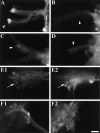
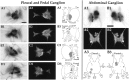
To identify neuropeptides that have a broad spectrum of actions on the feeding system of Aplysia, we searched for bioactive peptides that are present in both the gut and the CNS. We identified a family of structurally related nonapeptides and decapeptides (enterins) that are present in the gut and CNS of Aplysia, and most of which share the HSFVamide sequence at the C terminus. The structure of the enterin precursor deduced from cDNA cloning predicts 35 copies of 20 different enterins. Northern analysis, in situ hybridization, and immunocytochemistry show that the enterins are abundantly present in the CNS and the gut of Aplysia. Using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry we characterized the enterin-precursor processing, demonstrated that all of the precursor-predicted enterins are present, and determined post-translational modifications of various enterins. Enterin-positive neuronal somata and processes were found in the gut, and enterins inhibited contractions of the gut. In the CNS, the cerebral and buccal ganglia, which control feeding, contained the enterins. Enterin was also present in the nerve that connects these two ganglia. Enterins reduced the firing of interneurons B4/5 during feeding motor programs. Such enterin-induced reduction of firing also occurred when excitability of B4/5 was tested directly. Because reduction of B4/5 activity corresponds to a switch from egestive to ingestive behaviors, enterin may contribute to such program switching. Furthermore, because enterins are present throughout the nervous system, they may also play a regulatory role in nonfeeding behaviors of Aplysia.
Figures

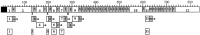



Similar articles
-
Identification and characterization of the feeding circuit-activating peptides, a novel neuropeptide family of aplysia.J Neurosci. 2002 Sep 1;22(17):7797-808. doi: 10.1523/JNEUROSCI.22-17-07797.2002. J Neurosci. 2002. PMID: 12196603 Free PMC article.
-
The enterins inhibit the contractile activity of the anterior aorta of Aplysia kurodai.J Exp Biol. 2002 Nov;205(Pt 22):3525-33. doi: 10.1242/jeb.205.22.3525. J Exp Biol. 2002. PMID: 12364405
-
PRQFVamide, a novel pentapeptide identified from the CNS and gut of Aplysia.J Neurophysiol. 2003 Jun;89(6):3114-27. doi: 10.1152/jn.00014.2003. Epub 2003 Feb 26. J Neurophysiol. 2003. PMID: 12612009
-
Physiology and biochemistry of peptidergic cotransmission in Aplysia.J Physiol Paris. 1993;87(3):141-51. doi: 10.1016/0928-4257(93)90025-o. J Physiol Paris. 1993. PMID: 7907908 Review.
-
Multifaceted Expression of Peptidergic Modulation in the Feeding System of Aplysia.ACS Chem Neurosci. 2018 Aug 15;9(8):1917-1927. doi: 10.1021/acschemneuro.7b00447. Epub 2018 Jan 24. ACS Chem Neurosci. 2018. PMID: 29309115 Free PMC article. Review.
Cited by
-
Profiling 26,000 Aplysia californica neurons by single cell mass spectrometry reveals neuronal populations with distinct neuropeptide profiles.J Biol Chem. 2022 Aug;298(8):102254. doi: 10.1016/j.jbc.2022.102254. Epub 2022 Jul 11. J Biol Chem. 2022. PMID: 35835221 Free PMC article.
-
Newly Identified Aplysia SPTR-Gene Family-Derived Peptides: Localization and Function.ACS Chem Neurosci. 2018 Aug 15;9(8):2041-2053. doi: 10.1021/acschemneuro.7b00513. Epub 2018 Mar 27. ACS Chem Neurosci. 2018. PMID: 29543430 Free PMC article.
-
Complementary interactions between command-like interneurons that function to activate and specify motor programs.J Neurosci. 2014 May 7;34(19):6510-21. doi: 10.1523/JNEUROSCI.5094-13.2014. J Neurosci. 2014. PMID: 24806677 Free PMC article.
-
Greenlip Abalone (Haliotis laevigata) Genome and Protein Analysis Provides Insights into Maturation and Spawning.G3 (Bethesda). 2019 Oct 7;9(10):3067-3078. doi: 10.1534/g3.119.400388. G3 (Bethesda). 2019. PMID: 31413154 Free PMC article.
-
Neural analog of arousal: persistent conditional activation of a feeding modulator by serotonergic initiators of locomotion.J Neurosci. 2008 Nov 19;28(47):12349-61. doi: 10.1523/JNEUROSCI.3855-08.2008. J Neurosci. 2008. PMID: 19020028 Free PMC article.
References
-
- Bateman A, Solomon S, Bennett HP. Post-translational modification of bovine pro-opiomelanocortin. Tyrosine sulfation and pyroglutamate formation, a mass spectrometric study. J Biol Chem. 1990;265:22130–22136. - PubMed
-
- Brezden BL, Yeoman MS, Gardner DR, Benjamin PR. FMRFamide-activated Ca2+ channels in Lymnaea heart cells are modulated by “SEEPLY,” a neuropeptide encoded on the same gene. J Neurophysiol. 1999;81:1818–1826. - PubMed
-
- Brezina V, Weiss KR. Analyzing the functional consequences of transmitter complexity. Trends Neurosci. 1997;20:538–543. - PubMed
-
- Brunet JF, Shapiro E, Foster SA, Kandel ER, Iino Y. Identification of a peptide specific for Aplysia sensory neurons by PCR-based differential screening. Science. 1991;252:856–859. - PubMed
-
- Busby WH, Quackenbush GE, Humm J, Youngblood WW, Kizer JS. An enzyme(s) that converts glutaminyl-peptides into pyroglutamyl-peptides. Presence in pituitary, brain, adrenal medulla, and lymphocytes. J Biol Chem. 1987;262:8532–8536. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
