Functional retinotopy of monkey visual cortex
- PMID: 11588200
- PMCID: PMC6763878
- DOI: 10.1523/JNEUROSCI.21-20-08286.2001
Functional retinotopy of monkey visual cortex
Abstract
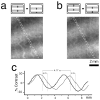
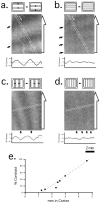
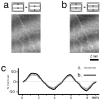
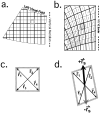
The operations of primary visual cortex generate continuous representations of orientation, ocular dominance, and retinotopy that, to fit in two dimensions, organize at separate but overlapping scales (e.g., 20-500 microm, 200 microm to 5 mm, and 2-33 mm). Where their scales overlap, these organizations interact; iso-orientation contours cross ocular dominance columns at right angles, and ocular dominance columns distort retinotopy near the V1/V2 border. To explore these interactions, we developed an optical technique for visualizing retinotopy in vivo that allows us to analyze it in relation to ocular dominance and orientation patterns. Our results show local retinotopic distortions in every region of macaque V1 that we examine, including regions far from the V1/V2 border. They also show a consistent relation between local axes of distortion and ocular dominance slabs, which they intersect at angles of approximately 90 degrees. A further correlation is provided by retinotopic maps from New World primates that show less distortion (9 vs 60%) in two species characterized by an absence of pronounced ocular dominance columns. Retinotopic maps from these New World primates also revealed an unexpected tilt of the vertical midline representation that diverged from the V1/V2 border by an angle of approximately 20 degrees. Overall, these results suggest a general tendency for slab-based organizations to distort retinotopy by representing the same part of space more than once in adjacent slabs.
Figures










References
-
- Allman JM, Kaas JH. Representations of the visual field in striate and adjoining cortex of the owl monkey (Aotus trivirgatus). Brain Res. 1971;35:89–106. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
