Cell adhesion and signaling on the fibronectin 1st type III repeat; requisite roles for cell surface proteoglycans and integrins
- PMID: 11591215
- PMCID: PMC57736
- DOI: 10.1186/1471-2121-2-18
Cell adhesion and signaling on the fibronectin 1st type III repeat; requisite roles for cell surface proteoglycans and integrins
Abstract
Background: The first type III repeat of fibronectin is known to be involved in fibronectin matrix assembly, and recombinant proteins from this type III repeat can inhibit cell proliferation, tumor metastasis and angiogenesis. We have analyzed the way rat aortic smooth muscle cells (RASMCs) interact with a recombinant protein encompassing a C-terminal portion of the first type III repeat of fibronectin (protein III1-C).
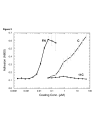
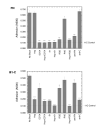
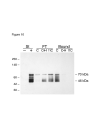
Results: Cells are able to adhere to and spread on III1-C coated on a dish. Both beta1 integrins and cell surface heparan sulfate proteoglycans serve as receptors for III1-C. For example, cell attachment to III1-C is partially inhibited by agents that block beta1 integrins or by heparin. Complete inhibition of cell attachment is seen only when integrin blocking agents are combined with heparin. Affinity chromatography revealed the binding of proteins that likely represent the integrin beta1 and alpha5 submits to a III1-C column. Cell adhesion to III1-C results in robust ERK1/2 activation that is blocked by integrin-blocking agents. In addition, cell adhesion to III1-C and ERK1/2 activation by III1-C are both inhibited by heparan sulfate but not by chondroitin sulfate. Moreover, heparitinase treatment, but not chondroitinase treatment of RASMCs results in reduced cell adhesion and ERK1/2 activation. Affinity chromatography experiments demonstrated that 35SO4-labeled cell surface heparan sulfate proteoglycans bound specifically to III1-C.
Conclusions: The results suggest that the 1st type III repeat of fibronectin contains a previously unrecognized cell adhesion domain that stimulates robust ERK1/2 activation in RASMCs. Cells interact with this domain through cell surface heparan sulfate proteoglycans and integrins, and both classes of receptors are required for optimal cell adhesion and ERK1/2 activation.
Figures




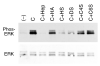


Similar articles
-
Participation of syndecan 2 in the induction of stress fiber formation in cooperation with integrin alpha5beta1: structural characteristics of heparan sulfate chains with avidity to COOH-terminal heparin-binding domain of fibronectin.Exp Cell Res. 2000 May 1;256(2):434-44. doi: 10.1006/excr.2000.4802. Exp Cell Res. 2000. PMID: 10772816
-
Identification of a novel heparin-binding site in the alternatively spliced IIICS region of fibronectin: roles of integrins and proteoglycans in cell adhesion to fibronectin splice variants.Matrix Biol. 2001 Feb;20(1):63-73. doi: 10.1016/s0945-053x(00)00131-1. Matrix Biol. 2001. PMID: 11246004
-
Inhibition of vascular smooth muscle cell growth by inhibition of fibronectin matrix assembly.Circ Res. 1998 Mar 23;82(5):548-56. doi: 10.1161/01.res.82.5.548. Circ Res. 1998. PMID: 9529159
-
Specific structural features of syndecans and heparan sulfate chains are needed for cell signaling.Braz J Med Biol Res. 2006 Feb;39(2):157-67. doi: 10.1590/s0100-879x2006000200001. Epub 2006 Feb 2. Braz J Med Biol Res. 2006. PMID: 16470302 Review.
-
Integrins in cell adhesion and signaling.Hum Cell. 1996 Sep;9(3):181-6. Hum Cell. 1996. PMID: 9183647 Review.
Cited by
-
Matricryptins Network with Matricellular Receptors at the Surface of Endothelial and Tumor Cells.Front Pharmacol. 2016 Feb 4;7:11. doi: 10.3389/fphar.2016.00011. eCollection 2016. Front Pharmacol. 2016. PMID: 26869928 Free PMC article. Review.
-
A gene expression-based comparison of cell adhesion to extracellular matrix and RGD-terminated monolayers.Biomaterials. 2015 Jun;52:385-94. doi: 10.1016/j.biomaterials.2015.02.045. Epub 2015 Mar 3. Biomaterials. 2015. PMID: 25818445 Free PMC article.
-
Charting the unexplored extracellular matrix in cancer.Int J Exp Pathol. 2018 Apr;99(2):58-76. doi: 10.1111/iep.12269. Epub 2018 Apr 19. Int J Exp Pathol. 2018. PMID: 29671911 Free PMC article. Review.
-
Anastellin, the angiostatic fibronectin peptide, is a selective inhibitor of lysophospholipid signaling.Mol Cancer Res. 2009 Feb;7(2):255-65. doi: 10.1158/1541-7786.MCR-08-0195. Epub 2009 Feb 10. Mol Cancer Res. 2009. PMID: 19208746 Free PMC article.
-
Display of cell surface sites for fibronectin assembly is modulated by cell adherence to (1)F3 and C-terminal modules of fibronectin.PLoS One. 2009;4(1):e4113. doi: 10.1371/journal.pone.0004113. Epub 2009 Jan 1. PLoS One. 2009. PMID: 19119318 Free PMC article.
References
-
- Hynes RO. Fibronectins New York: Springer-Verlag; 1990.
-
- Mercurius KO, Morla AO. Inhibition of vascular smooth muscle cell growth by inhibition of fibronectin matrix assembly. Circ Res. 1998;82:548–556. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

