Incomplete evidence: the inadequacy of databases in tracing published adverse drug reactions in clinical trials
- PMID: 11591220
- PMCID: PMC57741
- DOI: 10.1186/1471-2288-1-7
Incomplete evidence: the inadequacy of databases in tracing published adverse drug reactions in clinical trials
Abstract
Background: We would expect information on adverse drug reactions in randomised clinical trials to be easily retrievable from specific searches of electronic databases. However, complete retrieval of such information may not be straightforward, for two reasons. First, not all clinical drug trials provide data on the frequency of adverse effects. Secondly, not all electronic records of trials include terms in the abstract or indexing fields that enable us to select those with adverse effects data. We have determined how often automated search methods, using indexing terms and/or textwords in the title or abstract, would fail to retrieve trials with adverse effects data.
Methods: We used a sample set of 107 trials known to report frequencies of adverse drug effects, and measured the proportion that (i) were not assigned the appropriate adverse effects indexing terms in the electronic databases, and (ii) did not contain identifiable adverse effects textwords in the title or abstract.
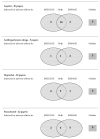
Results: Of the 81 trials with records on both MEDLINE and EMBASE, 25 were not indexed for adverse effects in either database. Twenty-six trials were indexed in one database but not the other. Only 66 of the 107 trials reporting adverse effects data mentioned this in the abstract or title of the paper. Simultaneous use of textword and indexing terms retrieved only 82/107 (77%) papers.
Conclusions: Specific search strategies based on adverse effects textwords and indexing terms will fail to identify nearly a quarter of trials that report on the rate of drug adverse effects.
Figures
References
-
- Loke YK, Derry S. A systematic review of the benefits and harms of antihypertensive drug therapy. Hypertension. 1999;34:710.
-
- Collins SL, Moore RA, McQuay HJ, Wiffen PJ, Edwards JE. Single dose oral ibuprofen and diclofenac for postoperative pain (Cochrane Review). The Cochrane Library, Issue 3, 2000. Oxford: Update Software. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources