Annotation transfer for genomics: measuring functional divergence in multi-domain proteins
- PMID: 11591640
- PMCID: PMC311165
- DOI: 10.1101/gr.183801
Annotation transfer for genomics: measuring functional divergence in multi-domain proteins
Abstract
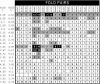
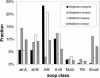
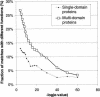
Annotation transfer is a principal process in genome annotation. It involves "transferring" structural and functional annotation to uncharacterized open reading frames (ORFs) in a newly completed genome from experimentally characterized proteins similar in sequence. To prevent errors in genome annotation, it is important that this process be robust and statistically well-characterized, especially with regard to how it depends on the degree of sequence similarity. Previously, we and others have analyzed annotation transfer in single-domain proteins. Multi-domain proteins, which make up the bulk of the ORFs in eukaryotic genomes, present more complex issues in functional conservation. Here we present a large-scale survey of annotation transfer in these proteins, using scop superfamilies to define domain folds and a thesaurus based on SWISS-PROT keywords to define functional categories. Our survey reveals that multi-domain proteins have significantly less functional conservation than single-domain ones, except when they share the exact same combination of domain folds. In particular, we find that for multi-domain proteins, approximate function can be accurately transferred with only 35% certainty for pairs of proteins sharing one structural superfamily. In contrast, this value is 67% for pairs of single-domain proteins sharing the same structural superfamily. On the other hand, if two multi-domain proteins contain the same combination of two structural superfamilies the probability of their sharing the same function increases to 80% in the case of complete coverage along the full length of both proteins, this value increases further to > 90%. Moreover, we found that only 70 of the current total of 455 structural superfamilies are found in both single and multi-domain proteins and only 14 of these were associated with the same function in both categories of proteins. We also investigated the degree to which function could be transferred between pairs of multi-domain proteins with respect to the degree of sequence similarity between them, finding that functional divergence at a given amount of sequence similarity is always about two-fold greater for pairs of multi-domain proteins (sharing similarity over a single domain) in comparison to pairs of single-domain ones, though the overall shape of the relationship is quite similar. Further information is available at http://partslist.org/func or http://bioinfo.mbb.yale.edu/partslist/func.
Figures

Similar articles
-
Assessing annotation transfer for genomics: quantifying the relations between protein sequence, structure and function through traditional and probabilistic scores.J Mol Biol. 2000 Mar 17;297(1):233-49. doi: 10.1006/jmbi.2000.3550. J Mol Biol. 2000. PMID: 10704319
-
PartsList: a web-based system for dynamically ranking protein folds based on disparate attributes, including whole-genome expression and interaction information.Nucleic Acids Res. 2001 Apr 15;29(8):1750-64. doi: 10.1093/nar/29.8.1750. Nucleic Acids Res. 2001. PMID: 11292848 Free PMC article.
-
Structural genomics analysis: characteristics of atypical, common, and horizontally transferred folds.Proteins. 2002 May 1;47(2):126-41. doi: 10.1002/prot.10078. Proteins. 2002. PMID: 11933060
-
Comparing genomes in terms of protein structure: surveys of a finite parts list.FEMS Microbiol Rev. 1998 Oct;22(4):277-304. doi: 10.1111/j.1574-6976.1998.tb00371.x. FEMS Microbiol Rev. 1998. PMID: 10357579 Review.
-
Reconsidering proteomic diversity with functional investigation of small ORFs and alternative ORFs.Exp Cell Res. 2020 Aug 1;393(1):112057. doi: 10.1016/j.yexcr.2020.112057. Epub 2020 May 6. Exp Cell Res. 2020. PMID: 32387289 Review.
Cited by
-
Bacterial regulatory networks are extremely flexible in evolution.Nucleic Acids Res. 2006 Jul 13;34(12):3434-45. doi: 10.1093/nar/gkl423. Print 2006. Nucleic Acids Res. 2006. PMID: 16840530 Free PMC article.
-
LigProf: a simple tool for in silico prediction of ligand-binding sites.J Mol Model. 2007 Mar;13(3):445-55. doi: 10.1007/s00894-006-0165-4. Epub 2007 Jan 3. J Mol Model. 2007. PMID: 17200839
-
Annotation transfer between genomes: protein-protein interologs and protein-DNA regulogs.Genome Res. 2004 Jun;14(6):1107-18. doi: 10.1101/gr.1774904. Genome Res. 2004. PMID: 15173116 Free PMC article.
-
Diversity in the architecture of ATLs, a family of plant ubiquitin-ligases, leads to recognition and targeting of substrates in different cellular environments.PLoS One. 2011;6(8):e23934. doi: 10.1371/journal.pone.0023934. Epub 2011 Aug 24. PLoS One. 2011. PMID: 21887349 Free PMC article.
-
Analyses of domains and domain fusions in human proto-oncogenes.BMC Bioinformatics. 2009 Mar 17;10:88. doi: 10.1186/1471-2105-10-88. BMC Bioinformatics. 2009. PMID: 19292927 Free PMC article.
References
-
- Devos D, Valencia A. Practical limits of function prediction. Proteins. 2000;41:98–107. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
