Pseudomonas aeruginosa PAO1 kills Caenorhabditis elegans by cyanide poisoning
- PMID: 11591663
- PMCID: PMC100099
- DOI: 10.1128/JB.183.21.6207-6214.2001
Pseudomonas aeruginosa PAO1 kills Caenorhabditis elegans by cyanide poisoning
Abstract
In this report we describe experiments to investigate a simple virulence model in which Pseudomonas aeruginosa PAO1 rapidly paralyzes and kills the nematode Caenorhabditis elegans. Our results imply that hydrogen cyanide is the sole or primary toxic factor produced by P. aeruginosa that is responsible for killing of the nematode. Four lines of evidence support this conclusion. First, a transposon insertion mutation in a gene encoding a subunit of hydrogen cyanide synthase (hcnC) eliminated nematode killing. Second, the 17 avirulent mutants examined all exhibited reduced cyanide synthesis, and the residual production levels correlated with killing efficiency. Third, exposure to exogenous cyanide alone at levels comparable to the level produced by PAO1 killed nematodes with kinetics similar to those observed with bacteria. The killing was not enhanced if hcnC mutant bacteria were present during cyanide exposure. And fourth, a nematode mutant (egl-9) resistant to P. aeruginosa was also resistant to killing by exogenous cyanide in the absence of bacteria. A model for nematode killing based on inhibition of mitochondrial cytochrome oxidase is presented. The action of cyanide helps account for the unusually broad host range of virulence of P. aeruginosa and may contribute to the pathogenesis in opportunistic human infections due to the bacterium.
Figures
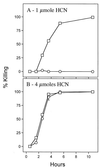
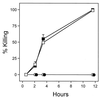
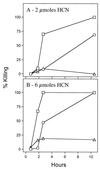
References
-
- Akerley B J, Miller J F. Understanding signal transduction during bacterial infection. Trends Microbiol. 1996;4:141–146. - PubMed
-
- Aravind L, Koonin E V. The DNA-repair protein AlkB, EGL-9, and leprecan define new families of 2-oxoglutarate- and iron-dependent dioxygenases. Genome Biol. Res. Vol. 2. 2001. http://genomebiology.com/2001/2/3/research/0007/ [Online.] http://genomebiology.com/2001/2/3/research/0007/. . - PMC - PubMed
-
- Blumer C, Haas D. Mechanism, regulation, and ecological role of bacterial cyanide biosynthesis. Arch Microbiol. 2000;173:170–177. - PubMed
-
- Boman H G, Nilsson I, Rasmuson B. Inducible antibacterial defence system in Drosophila. Nature. 1972;237:232–235. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

