An archaeal photosignal-transducing module mediates phototaxis in Escherichia coli
- PMID: 11591681
- PMCID: PMC100132
- DOI: 10.1128/JB.183.21.6365-6371.2001
An archaeal photosignal-transducing module mediates phototaxis in Escherichia coli
Abstract
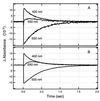
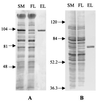
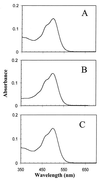
Halophilic archaea, such as Halobacterium salinarum and Natronobacterium pharaonis, alter their swimming behavior by phototaxis responses to changes in light intensity and color using visual pigment-like sensory rhodopsins (SRs). In N. pharaonis, SRII (NpSRII) mediates photorepellent responses through its transducer protein, NpHtrII. Here we report the expression of fusions of NpSRII and NpHtrII and fusion hybrids with eubacterial cytoplasmic domains and analyze their function in vivo in haloarchaea and in eubacteria. A fusion in which the C terminus of NpSRII is connected by a short flexible linker to NpHtrII is active in phototaxis signaling for H. salinarum, showing that the fusion does not inhibit functional receptor-transducer interactions. We replaced the cytoplasmic portions of this fusion protein with the cytoplasmic domains of Tar and Tsr, chemotaxis transducers from enteric eubacteria. Purification of the fusion protein from H. salinarum and Tar fusion chimera from Escherichia coli membranes shows that the proteins are not cleaved and exhibit absorption spectra characteristic of wild-type membranes. Their photochemical reaction cycles in H. salinarum and E. coli membranes, respectively, are similar to those of native NpSRII in N. pharaonis. These fusion chimeras mediate retinal-dependent phototaxis responses by Escherichia coli, establishing that the nine-helix membrane portion of the receptor-transducer complex is a modular functional unit able to signal in heterologous membranes. This result confirms a current model for SR-Htr signal transduction in which the Htr transducers are proposed to interact physically and functionally with their cognate sensory rhodopsins via helix-helix contacts between their transmembrane segments.
Figures




References
-
- Aravind L, Ponting C P. The cytoplasmic helical linker domain of receptor histidine kinase and methyl-accepting proteins is common to many prokaryotic signalling proteins. FEMS Microbiol Lett. 1999;176:111–116. - PubMed
-
- Baumgartner J W, Kim C, Brissette R E, Inouye M, Park C, Hazelbauer G L. Transmembrane signalling by a hybrid protein: communication from the domain of chemoreceptor Trg that recognizes sugar-binding proteins to the kinase/phosphatase domain of osmosensor EnvZ. J Bacteriol. 1994;176:1157–1163. - PMC - PubMed
-
- Bivin D B, Stoeckenius W. Photoactive retinal pigments in haloalkaliphilic bacteria. J Gen Microbiol. 1986;132(Pt. 8):2167–2177. - PubMed
-
- Bogomolni R A, Spudich J L. Spectroscopic assays for sensory rhodopsin I and II in Halobacterium salinarium cells and enriched membrane preparations. In: Robb F T, Place A R, Sowers K R, Schreier H J, DasSarma S, Fleischmann E M, editors. Archaea: a laboratory manual. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1995. pp. 63–73.
-
- Elish M E, Pierce J R, Earhart C F. Biochemical analysis of spontaneous fepA mutants of Escherichia coli. J Gen Microbiol. 1988;134(Pt. 5):1355–1364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

