A high-speed atomic force microscope for studying biological macromolecules
- PMID: 11592975
- PMCID: PMC60077
- DOI: 10.1073/pnas.211400898
A high-speed atomic force microscope for studying biological macromolecules
Abstract
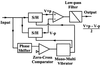
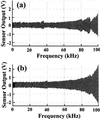
The atomic force microscope (AFM) is a powerful tool for imaging individual biological molecules attached to a substrate and placed in aqueous solution. At present, however, it is limited by the speed at which it can successively record highly resolved images. We sought to increase markedly the scan speed of the AFM, so that in the future it can be used to study the dynamic behavior of biomolecules. For this purpose, we have developed a high-speed scanner, free of resonant vibrations up to 60 kHz, small cantilevers with high resonance frequencies (450-650 kHz) and small spring constants (150-280 pN/nm), an objective-lens type of deflection detection device, and several electronic devices of wide bandwidth. Integration of these various devices has produced an AFM that can capture a 100 x 100 pixel(2) image within 80 ms and therefore can generate a movie consisting of many successive images (80-ms intervals) of a sample in aqueous solution. This is demonstrated by imaging myosin V molecules moving on mica (see http://www.s.kanazawa-u.ac.jp/phys/biophys/bmv_movie.htm).
Figures



References
-
- Binnig G, Quate C F, Gerber C. Phys Rev Lett. 1986;56:930–933. - PubMed
-
- Drake B, Prater C B, Weisenhorn A L, Gould S A, Albrecht T R, Quate C F, Cannell D S, Hansma H G, Hansma P K. Science. 1989;243:1586–1589. - PubMed
-
- Bustamante C, Rivetti C, Keller D J. Curr Opin Struct Biol. 1997;7:709–716. - PubMed
-
- Kassas S, Thomson N H, Smith B L, Hansma H G, Zhu X, Guthold M, Bustamante C, Kool E T, Kashlev M, Hansma P K. Biochemistry. 1997;36:461–468. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

