Hierarchical self-assembly of chiral rod-like molecules as a model for peptide beta -sheet tapes, ribbons, fibrils, and fibers
- PMID: 11592996
- PMCID: PMC59814
- DOI: 10.1073/pnas.191250198
Hierarchical self-assembly of chiral rod-like molecules as a model for peptide beta -sheet tapes, ribbons, fibrils, and fibers
Abstract
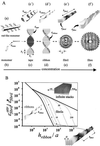
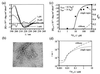
A generic statistical mechanical model is presented for the self-assembly of chiral rod-like units, such as beta-sheet-forming peptides, into helical tapes, which with increasing concentration associate into twisted ribbons (double tapes), fibrils (twisted stacks of ribbons), and fibers (entwined fibrils). The finite fibril width and helicity is shown to stem from a competition between the free energy gain from attraction between ribbons and the penalty because of elastic distortion of the intrinsically twisted ribbons on incorporation into a growing fibril. Fibers are stabilized similarly. The behavior of two rationally designed 11-aa residue peptides, P(11)-I and P(11)-II, is illustrative of the proposed scheme. P(11)-I and P(11)-II are designed to adopt the beta-strand conformation and to self-assemble in one dimension to form antiparallel beta-sheet tapes, ribbons, fibrils, and fibers in well-defined solution conditions. The energetic parameters governing self-assembly have been estimated from the experimental data using the model. The 8-nm-wide fibrils consist of eight tapes, are extremely robust (scission energy approximately 200 k(B)T), and sufficiently rigid (persistence length l(fibril) approximately 20-70 microm) to form nematic solutions at peptide concentration c approximately 0.9 mM (volume fraction approximately 0.0009 vol/vol), which convert to self-supporting nematic gels at c > 4 mM. More generally, these observations provide a new insight into the generic self-assembling properties of beta-sheet-forming peptides and shed new light on the factors governing the structures and stability of pathological amyloid fibrils in vivo. The model also provides a prescription of routes to novel macromolecules based on a variety of self-assembling chiral units, and protocols for extraction of the associated energy changes.
Figures

References
-
- Artsaenko O, Kettig B, Fiedler K, Conrad K, During K. Mol Breeding. 1998;4:313–319.
-
- Urry D. Trend Biotechnol. 1999;17:249–257. - PubMed
-
- Panitch A, Yamaoka T, Fournier M J, Mason T L, Tirrell D A. Macromolecules. 1999;32:1701–1703.
-
- Krejchi M T, Atkins E D T, Waddon A J, Fournier J, Mason T L, Tirrell D A. Science. 1994;265:1427–1432. - PubMed
-
- Zhang S G, Altman M. React Funct Polym. 1999;41:91–102.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

