A tumor necrosis factor alpha- and interleukin 6-inducible protein that interacts with the small subunit of DNA polymerase delta and proliferating cell nuclear antigen
- PMID: 11593007
- PMCID: PMC59753
- DOI: 10.1073/pnas.221452098
A tumor necrosis factor alpha- and interleukin 6-inducible protein that interacts with the small subunit of DNA polymerase delta and proliferating cell nuclear antigen
Abstract
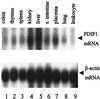
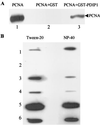
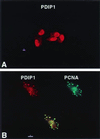
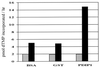
A cDNA encoding a protein of 36 kDa, polymerase delta-interacting protein 1 (PDIP1), that interacts with the small subunit (p50) of DNA polymerase delta (pol delta) was identified in a two-hybrid screen of a HepG2 cDNA library by using p50 as bait. The interaction of PDIP1 with p50 was confirmed by pull-down assays, and a similar assay was used to demonstrate that PDIP1 interacts directly with the proliferating cell nuclear antigen (PCNA). PCNA and p50 bound to PDIP1 simultaneously, and PDIP1 stimulated pol delta activity in vitro in the presence, but not the absence, of PCNA, suggesting that PDIP1 also interacts functionally with both p50 and PCNA. Subcellular localization studies demonstrated that PDIP1 is a nuclear protein that colocalizes with PCNA at replication foci. A putative PCNA-binding motif was identified within the C terminus of PDIP1, and a synthetic peptide containing this PCNA-binding motif was shown to bind PCNA by far-Western analysis. Northern analysis demonstrated that PDIP1 mRNA is present in a wide variety of human tissues. PDIP1 was found to be highly homologous to a previously identified protein, B12 [Wolf, F. W., Marks, R. M., Sarma. V., Byers, M. G., Katz, R. W., Shows, T. B. & Dixit, V. M. (1992) J. Biol. Chem. 267, 1317-1326], one of the early response genes induced by tumor necrosis factor alpha. PDIP1 synthesis can also be induced by tumor necrosis factor alpha and by IL-6, cytokines essential for liver regeneration after loss of hepatic tissue. It is suggested that PDIP1 provides a link between cytokine activation and DNA replication in liver as well as in other tissues.
Figures



Similar articles
-
Cloning of two rat PDIP1 related genes and their interactions with proliferating cell nuclear antigen.J Exp Zool A Comp Exp Biol. 2005 Mar 1;303(3):227-40. doi: 10.1002/jez.a.150. J Exp Zool A Comp Exp Biol. 2005. PMID: 15726626
-
A novel PDIP1-related protein, KCTD10, that interacts with proliferating cell nuclear antigen and DNA polymerase delta.Biochim Biophys Acta. 2005 Jul 10;1729(3):200-3. doi: 10.1016/j.bbaexp.2005.05.005. Biochim Biophys Acta. 2005. PMID: 15982757
-
Direct interaction of proliferating cell nuclear antigen with the small subunit of DNA polymerase delta.J Biol Chem. 2002 Jul 5;277(27):24340-5. doi: 10.1074/jbc.M200065200. Epub 2002 May 1. J Biol Chem. 2002. PMID: 11986310
-
Human PCNA Structure, Function and Interactions.Biomolecules. 2020 Apr 8;10(4):570. doi: 10.3390/biom10040570. Biomolecules. 2020. PMID: 32276417 Free PMC article. Review.
-
Proliferating cell nuclear antigen: a proteomics view.Cell Mol Life Sci. 2008 Nov;65(23):3789-808. doi: 10.1007/s00018-008-8305-x. Cell Mol Life Sci. 2008. PMID: 18726183 Free PMC article. Review.
Cited by
-
Genetic and Epigenetic Etiology Underlying Autism Spectrum Disorder.J Clin Med. 2020 Mar 31;9(4):966. doi: 10.3390/jcm9040966. J Clin Med. 2020. PMID: 32244359 Free PMC article. Review.
-
KCTD10 is involved in the cardiovascular system and Notch signaling during early embryonic development.PLoS One. 2014 Nov 17;9(11):e112275. doi: 10.1371/journal.pone.0112275. eCollection 2014. PLoS One. 2014. PMID: 25401743 Free PMC article.
-
The polymerase δ-interacting protein family and their emerging roles in diseases.Front Med (Lausanne). 2022 Nov 8;9:1026931. doi: 10.3389/fmed.2022.1026931. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36425112 Free PMC article. Review.
-
CRISPR/Cas9-mediated Knockout of the Neuropsychiatric Risk Gene KCTD13 Causes Developmental Deficits in Human Cortical Neurons Derived from Induced Pluripotent Stem Cells.Mol Neurobiol. 2020 Feb;57(2):616-634. doi: 10.1007/s12035-019-01727-1. Epub 2019 Aug 11. Mol Neurobiol. 2020. PMID: 31402430
-
Androgen receptor ubiquitination links KCTD13 to genitourinary tract defects.FASEB J. 2025 Feb 28;39(4):e70406. doi: 10.1096/fj.202402072RR. FASEB J. 2025. PMID: 39968753
References
-
- Warbrick E. BioEssays. 2000;22:997–1006. - PubMed
-
- Tsurimoto T. Front Biosci. 1999;4:D849–D858. - PubMed
-
- Warbrick E. BioEssays. 1998;20:195–199. - PubMed
-
- Gulbis J M, Kelman Z, Hurwitz J, O'Donnell M, Kuriyan J. Cell. 1996;87:297–306. - PubMed
-
- Waga S, Stillman B. Annu Rev Biochem. 1998;67:721–751. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

