Functional analysis of mutant and wild-type Drosophila origin recognition complex
- PMID: 11593009
- PMCID: PMC59756
- DOI: 10.1073/pnas.211342798
Functional analysis of mutant and wild-type Drosophila origin recognition complex
Abstract
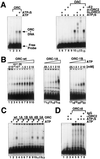
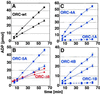
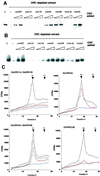
The origin recognition complex (ORC) is the DNA replication initiator protein in eukaryotes. We have reconstituted a functional recombinant Drosophila ORC and compared activities of the wild-type and several mutant ORC variants. Drosophila ORC is an ATPase, and our studies show that the ORC1 subunit is essential for ATP hydrolysis and for ATP-dependent DNA binding. Moreover, DNA binding by ORC reduces its ATP hydrolysis activity. In vitro, ORC binds to chromatin in an ATP-dependent manner, and this process depends on the functional AAA(+) nucleotide-binding domain of ORC1. Mutations in the ATP-binding domain of ORC1 are unable to support cell-free DNA replication. However, mutations in the putative ATP-binding domain of either the ORC4 or ORC5 subunits do not affect either of these functions. We also provide evidence that the Drosophila ORC6 subunit is directly required for all of these activities and that a large pool of ORC6 is present in the cytoplasm, cytologically proximal to the cell membrane. Studies reported here provide the first functional dissection of a metazoan initiator and highlight the basic conserved and divergent features among Drosophila and budding yeast ORC complexes.
Figures

Similar articles
-
Role of the Orc6 protein in origin recognition complex-dependent DNA binding and replication in Drosophila melanogaster.Mol Cell Biol. 2007 Apr;27(8):3143-53. doi: 10.1128/MCB.02382-06. Epub 2007 Feb 5. Mol Cell Biol. 2007. PMID: 17283052 Free PMC article.
-
Coordinate binding of ATP and origin DNA regulates the ATPase activity of the origin recognition complex.Cell. 1997 Feb 21;88(4):493-502. doi: 10.1016/s0092-8674(00)81889-9. Cell. 1997. PMID: 9038340
-
ATP hydrolysis by ORC catalyzes reiterative Mcm2-7 assembly at a defined origin of replication.Mol Cell. 2004 Dec 22;16(6):967-78. doi: 10.1016/j.molcel.2004.11.038. Mol Cell. 2004. PMID: 15610739
-
The origin recognition complex protein family.Genome Biol. 2009;10(3):214. doi: 10.1186/gb-2009-10-3-214. Epub 2009 Mar 17. Genome Biol. 2009. PMID: 19344485 Free PMC article. Review.
-
DnaA, ORC, and Cdc6: similarity beyond the domains of life and diversity.Biochem Cell Biol. 2010 Feb;88(1):49-62. doi: 10.1139/o09-154. Biochem Cell Biol. 2010. PMID: 20130679 Review.
Cited by
-
A human cancer cell line initiates DNA replication normally in the absence of ORC5 and ORC2 proteins.J Biol Chem. 2020 Dec 11;295(50):16949-16959. doi: 10.1074/jbc.RA120.015450. Epub 2020 Sep 28. J Biol Chem. 2020. PMID: 32989049 Free PMC article.
-
Site-specific interaction of the murine pre-replicative complex with origin DNA: assembly and disassembly during cell cycle transit and differentiation.Nucleic Acids Res. 2007;35(20):6701-13. doi: 10.1093/nar/gkm555. Epub 2007 Oct 4. Nucleic Acids Res. 2007. PMID: 17916579 Free PMC article.
-
A new class of disordered elements controls DNA replication through initiator self-assembly.Elife. 2019 Sep 27;8:e48562. doi: 10.7554/eLife.48562. Elife. 2019. PMID: 31560342 Free PMC article.
-
Orc6 is required for dynamic recruitment of Cdt1 during repeated Mcm2-7 loading.Genes Dev. 2007 Nov 15;21(22):2897-907. doi: 10.1101/gad.1596807. Genes Dev. 2007. PMID: 18006685 Free PMC article.
-
Functional analysis of an Orc6 mutant in Drosophila.Proc Natl Acad Sci U S A. 2009 Jun 30;106(26):10672-7. doi: 10.1073/pnas.0902670106. Epub 2009 Jun 16. Proc Natl Acad Sci U S A. 2009. PMID: 19541634 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

