Proteomic method identifies proteins nitrated in vivo during inflammatory challenge
- PMID: 11593016
- PMCID: PMC59826
- DOI: 10.1073/pnas.221269198
Proteomic method identifies proteins nitrated in vivo during inflammatory challenge
Abstract
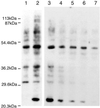
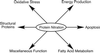
Inflammation in asthma, sepsis, transplant rejection, and many neurodegenerative diseases associates an up-regulation of NO synthesis with increased protein nitration at tyrosine. Nitration can cause protein dysfunction and is implicated in pathogenesis, but few proteins that appear nitrated in vivo have been identified. To understand how this modification impacts physiology and disease, we used a proteomic approach toward targets of protein nitration in both in vivo and cell culture inflammatory disease models. This approach identified more than 40 nitrotyrosine-immunopositive proteins, including 30 not previously identified, that became modified as a consequence of the inflammatory response. These targets include proteins involved in oxidative stress, apoptosis, ATP production, and other metabolic functions. Our approach provides a means toward obtaining a comprehensive view of the nitroproteome and promises to broaden understanding of how NO regulates cellular processes.
Figures


References
-
- Beckman J S, Koppenol W H. Am J Physiol. 1996;271:C1424–C1437. - PubMed
-
- Halliwell B, Zhao K, Whiteman M. Free Radical Res. 1999;31:651–669. - PubMed
-
- Ischiropoulos H. Arch Biochem Biophys. 1998;356:1–11. - PubMed
-
- Gole M D, Souza J M, Choi I, Hertkorn C, Malcolm S, Foust R F, III, Finkel B, Lanken P N, Ischiropoulos H. Am J Physiol. 2000;278:L961–L967. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

