Specific treatment of autoimmunity with recombinant invariant chains in which CLIP is replaced by self-epitopes
- PMID: 11593032
- PMCID: PMC59786
- DOI: 10.1073/pnas.221220998
Specific treatment of autoimmunity with recombinant invariant chains in which CLIP is replaced by self-epitopes
Abstract
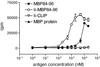
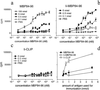
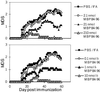
The invariant chain (Ii) binds to newly synthesized MHC class II molecules with the CLIP region of Ii occupying the peptide-binding groove. Here we demonstrate that recombinant Ii proteins with the CLIP region replaced by antigenic self-epitopes are highly efficient in activating and silencing specific T cells in vitro and in vivo. The Ii proteins require endogenous processing by antigen-presenting cells for efficient T cell activation. An Ii protein encompassing the epitope myelin basic protein amino acids 84-96 (Ii-MBP84-96) induced the model autoimmune disease experimental allergic encephalomyelitis (EAE) with a higher severity and earlier onset than the peptide. When applied in a tolerogenic manner, Ii-MBP84-96 abolished antigen-specific T cell proliferation and suppressed peptide-induced EAE more effectively than peptide alone. Importantly, i.v. administration of Ii proteins after EAE induction completely abrogated the disease, whereas peptides only marginally suppressed disease symptoms. Ii fusion proteins are thus more efficient than peptide in modulating CD4(+) T cell-mediated autoimmunity, documenting their superior qualities for therapeutic antigen delivery in vivo.
Figures



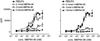
Similar articles
-
Efficient presentation of known HLA class II-restricted MAGE-A3 epitopes by dendritic cells electroporated with messenger RNA encoding an invariant chain with genetic exchange of class II-associated invariant chain peptide.Cancer Res. 2003 Sep 1;63(17):5587-94. Cancer Res. 2003. PMID: 14500399
-
Superior tumor protection induced by a cellular vaccine carrying a tumor-specific T helper epitope by genetic exchange of the class II-associated invariant chain peptide.Cancer Res. 2000 Nov 15;60(22):6427-33. Cancer Res. 2000. PMID: 11103809
-
Self and non-self peptides treat autoimmune encephalomyelitis: T cell anergy or competition for major histocompatibility complex class II binding?Eur J Immunol. 1995 Jul;25(7):2059-63. doi: 10.1002/eji.1830250738. Eur J Immunol. 1995. PMID: 7542603
-
On the initial trigger of myasthenia gravis and suppression of the disease by antibodies against the MHC peptide region involved in the presentation of a pathogenic T-cell epitope.Crit Rev Immunol. 2001;21(1-3):1-27. Crit Rev Immunol. 2001. PMID: 11642597 Review.
-
CD4+ T-cell activation for immunotherapy of malignancies using Ii-Key/MHC class II epitope hybrid vaccines.Vaccine. 2012 Apr 16;30(18):2805-10. doi: 10.1016/j.vaccine.2012.02.031. Epub 2012 Mar 3. Vaccine. 2012. PMID: 22386748 Review.
Cited by
-
Tolerance induction in experimental autoimmune encephalomyelitis using non-myeloablative hematopoietic gene therapy with autoantigen.Mol Ther. 2009 May;17(5):897-905. doi: 10.1038/mt.2009.42. Epub 2009 Mar 10. Mol Ther. 2009. PMID: 19277013 Free PMC article.
-
Prominent T-Cell Responses against the Acetylcholine Receptor ε Subunit in Myasthenia Gravis.Neurol Res Int. 2019 Mar 3;2019:1969068. doi: 10.1155/2019/1969068. eCollection 2019. Neurol Res Int. 2019. PMID: 30941215 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

