Cardiovascular, skeletal, and renal defects in mice with a targeted disruption of the Pkd1 gene
- PMID: 11593033
- PMCID: PMC59787
- DOI: 10.1073/pnas.211191098
Cardiovascular, skeletal, and renal defects in mice with a targeted disruption of the Pkd1 gene
Abstract
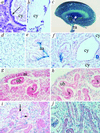
Autosomal dominant polycystic kidney disease (ADPKD) is characterized by cyst formation in the kidney, liver, and pancreas and is associated often with cardiovascular abnormalities such as hypertension, mitral valve prolapse, and intracranial aneurysms. It is caused by mutations in PKD1 or PKD2, encoding polycystin-1 and -2, which together form a cell surface nonselective cation ion channel. Pkd2-/- mice have cysts in the kidney and pancreas and defects in cardiac septation, whereas Pkd1(del34) -/- and Pkd1(L) -/- mice have cysts but no cardiac abnormalities, although vascular fragility was reported in the latter. Here we describe mice carrying a targeted mutation in Pkd1 (Pkd1(del17-21betageo)), which defines its expression pattern by using a lacZ reporter gene and may identify novel functions for polycystin-1. Although Pkd1(del17-21betageo) +/- adult mice develop renal and hepatic cysts, Pkd1(del17-21betageo) -/- embryos die at embryonic days 13.5-14.5 from a primary cardiovascular defect that includes double outflow right ventricle, disorganized myocardium, and abnormal atrio-ventricular septation. Skeletal development is also severely compromised. These abnormalities correlate with the major sites of Pkd1 expression. During nephrogenesis, Pkd1 is expressed in maturing tubular epithelial cells from embryonic day 15.5. This expression coincides with the onset of cyst formation in Pkd1(del34) -/-, Pkd1(L) -/-, and Pkd2-/- mice, supporting the hypothesis that polycystin-1 and polycystin-2 interact in vivo and that their failure to do so leads to abnormalities in tubule morphology and function.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

