Targeting tissue factor on tumor vascular endothelial cells and tumor cells for immunotherapy in mouse models of prostatic cancer
- PMID: 11593034
- PMCID: PMC59788
- DOI: 10.1073/pnas.201420298
Targeting tissue factor on tumor vascular endothelial cells and tumor cells for immunotherapy in mouse models of prostatic cancer
Abstract
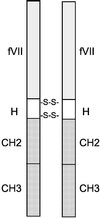
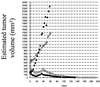
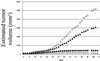
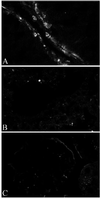
The efficacy and safety of an immunoconjugate (icon) molecule, composed of a mutated mouse factor VII (mfVII) targeting domain and the Fc effector domain of an IgG1 Ig (mfVII/Fc icon), was tested with a severe combined immunodeficient (SCID) mouse model of human prostatic cancer and an immunocompetent mouse model of mouse prostatic cancer. The SCID mice were first injected s.c. with a human prostatic tumor line, forming a skin tumor that produces a high blood titer of prostate-specific antigen and metastasizes to bone. The icon was encoded in a replication-incompetent adenoviral vector that was injected directly into the skin tumor. The tumor cells infected by the vector synthesize and secrete the icon into the blood, and the blood-borne icon binds with high affinity and specificity to mouse tissue factor expressed on endothelial cells lining the lumen of the tumor vasculature and to human tissue factor expressed on the tumor cells. The Fc domain of the icon activates a cytolytic immune attack against cells that bind the icon. The immunotherapy tests in SCID mice demonstrated that intratumoral injections of the adenoviral vector encoding the mfVII/human Fc icon resulted in long-term regression of the injected human prostatic tumor and also of a distant uninjected tumor, without associated toxicity to the mice. Comparable results were obtained with a SCID mouse model of human melanoma. At the end of the experiments the mice appeared to be free of viable tumor cells. This protocol also could be efficacious for treating cancer patients who have vascularized tumors.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

