Poly(ADP-ribose) glycohydrolase mediates oxidative and excitotoxic neuronal death
- PMID: 11593040
- PMCID: PMC59796
- DOI: 10.1073/pnas.211202598
Poly(ADP-ribose) glycohydrolase mediates oxidative and excitotoxic neuronal death
Abstract
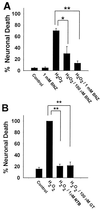
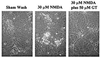
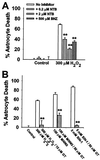
Excessive activation of poly(ADP-ribose) polymerase 1 (PARP1) leads to NAD(+) depletion and cell death during ischemia and other conditions that generate extensive DNA damage. When activated by DNA strand breaks, PARP1 uses NAD(+) as substrate to form ADP-ribose polymers on specific acceptor proteins. These polymers are in turn rapidly degraded by poly(ADP-ribose) glycohydrolase (PARG), a ubiquitously expressed exo- and endoglycohydrolase. In this study, we examined the role of PARG in the PARP1-mediated cell death pathway. Mouse neuron and astrocyte cultures were exposed to hydrogen peroxide, N-methyl-d-aspartate (NMDA), or the DNA alkylating agent, N-methyl-N'-nitro-N-nitrosoguanidine (MNNG). Cell death in each condition was markedly reduced by the PARP1 inhibitor benzamide and equally reduced by the PARG inhibitors gallotannin and nobotanin B. The PARP1 inhibitor benzamide and the PARG inhibitor gallotannin both prevented the NAD(+) depletion that otherwise results from PARP1 activation by MNNG or H(2)O(2). However, these agents had opposite effects on protein poly(ADP-ribosyl)ation. Immunostaining for poly(ADP-ribose) on Western blots and neuron cultures showed benzamide to decrease and gallotannin to increase poly(ADP-ribose) accumulation during MNNG exposure. These results suggest that PARG inhibitors do not inhibit PARP1 directly, but instead prevent PARP1-mediated cell death by slowing the turnover of poly(ADP-ribose) and thus slowing NAD(+) consumption. PARG appears to be a necessary component of the PARP-mediated cell death pathway, and PARG inhibitors may have promise as neuroprotective agents.
Figures
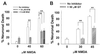
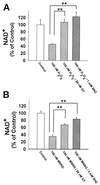


Similar articles
-
The poly(ADP-ribose) glycohydrolase inhibitor gallotannin blocks oxidative astrocyte death.Neuroreport. 2000 May 15;11(7):1385-8. doi: 10.1097/00001756-200005150-00007. Neuroreport. 2000. PMID: 10841343
-
Altered poly(ADP-ribose) metabolism impairs cellular responses to genotoxic stress in a hypomorphic mutant of poly(ADP-ribose) glycohydrolase.Exp Cell Res. 2007 Mar 10;313(5):984-96. doi: 10.1016/j.yexcr.2006.12.025. Epub 2007 Jan 10. Exp Cell Res. 2007. PMID: 17276427
-
Poly(ADP-ribose) glycohydrolase as a target for neuroprotective intervention: assessment of currently available pharmacological tools.Eur J Pharmacol. 2004 Aug 16;497(1):7-16. doi: 10.1016/j.ejphar.2004.06.042. Eur J Pharmacol. 2004. PMID: 15321729
-
New Insights into the Roles of NAD+-Poly(ADP-ribose) Metabolism and Poly(ADP-ribose) Glycohydrolase.Curr Protein Pept Sci. 2016;17(7):668-682. doi: 10.2174/1389203717666160419150014. Curr Protein Pept Sci. 2016. PMID: 27817743 Review.
-
Structure and function of poly(ADP-ribose) polymerase-1: role in oxidative stress-related pathologies.Curr Vasc Pharmacol. 2005 Jul;3(3):209-14. doi: 10.2174/1570161054368625. Curr Vasc Pharmacol. 2005. PMID: 16026317 Review.
Cited by
-
ADP-ribose hydrolases: biological functions and potential therapeutic targets.Expert Rev Mol Med. 2024 Oct 8;26:e21. doi: 10.1017/erm.2024.17. Expert Rev Mol Med. 2024. PMID: 39375922 Free PMC article. Review.
-
Frex and FrexH: Indicators of metabolic states in living cells.Bioeng Bugs. 2012 May-Jun;3(3):181-8. doi: 10.4161/bbug.19769. Epub 2012 May 1. Bioeng Bugs. 2012. PMID: 22572785 Free PMC article.
-
Using Fractional Intensities of Time-resolved Fluorescence to Sensitively Quantify NADH/NAD+ with Genetically Encoded Fluorescent Biosensors.Sci Rep. 2017 Jun 23;7(1):4209. doi: 10.1038/s41598-017-04051-7. Sci Rep. 2017. PMID: 28646144 Free PMC article.
-
Transcripts of damaged genes in the brain during cerebral oxidative stress.J Neurosci Res. 2002 Dec 15;70(6):713-20. doi: 10.1002/jnr.10454. J Neurosci Res. 2002. PMID: 12444593 Free PMC article. Review.
-
Poly (ADP-ribose) polymerase: An Overview of Mechanistic Approaches and Therapeutic Opportunities in the Management of Stroke.Neurochem Res. 2022 Jul;47(7):1830-1852. doi: 10.1007/s11064-022-03595-z. Epub 2022 Apr 18. Neurochem Res. 2022. PMID: 35437712 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

