The in vivo role of p38 MAP kinases in cardiac remodeling and restrictive cardiomyopathy
- PMID: 11593045
- PMCID: PMC59806
- DOI: 10.1073/pnas.211086598
The in vivo role of p38 MAP kinases in cardiac remodeling and restrictive cardiomyopathy
Abstract
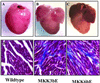
Stress-induced mitogen-activated protein kinase (MAP) p38 is activated in various forms of heart failure, yet its effects on the intact heart remain to be established. Targeted activation of p38 MAP kinase in ventricular myocytes was achieved in vivo by using a gene-switch transgenic strategy with activated mutants of upstream kinases MKK3bE and MKK6bE. Transgene expression resulted in significant induction of p38 kinase activity and premature death at 7-9 weeks. Both groups of transgenic hearts exhibited marked interstitial fibrosis and expression of fetal marker genes characteristic of cardiac failure, but no significant hypertrophy at the organ level. Echocardiographic and pressure-volume analyses revealed a similar extent of systolic contractile depression and restrictive diastolic abnormalities related to markedly increased passive chamber stiffness. However, MKK3bE-expressing hearts had increased end-systolic chamber volumes and a thinned ventricular wall, associated with heterogeneous myocyte atrophy, whereas MKK6bE hearts had reduced end-diastolic ventricular cavity size, a modest increase in myocyte size, and no significant myocyte atrophy. These data provide in vivo evidence for a negative inotropic and restrictive diastolic effect from p38 MAP kinase activation in ventricular myocytes and reveal specific roles of p38 pathway in the development of ventricular end-systolic remodeling.
Figures





References
-
- Cohn J N, Bristow M R, Chien K R, Colucci W S, Frazier O H, Leinwand L A, Lorell B H, Moss A J, Sonnenblick E H, Walsh R A, et al. Circulation. 1997;95:766–770. - PubMed
-
- Grossman W. Am J Med. 1980;69:576–584. - PubMed
-
- Chien K R, Grace A A. In: Heart Disease. Braunwald E, editor. Philadelphia: Saunders; 1997. pp. 1626–1649.
-
- Parmley W W. Clin Cardiol. 1992;15, Suppl. 1:I5–I12. - PubMed
-
- Chien K R. Cell. 1999;98:555–558. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

