Intranasal inoculation with the olfactory bulb line variant of mouse hepatitis virus causes extensive destruction of the olfactory bulb and accelerated turnover of neurons in the olfactory epithelium of mice
- PMID: 11595671
- PMCID: PMC7110028
- DOI: 10.1093/chemse/26.8.937
Intranasal inoculation with the olfactory bulb line variant of mouse hepatitis virus causes extensive destruction of the olfactory bulb and accelerated turnover of neurons in the olfactory epithelium of mice
Abstract
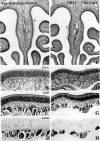
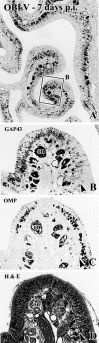
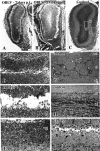
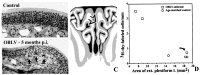
Viral upper respiratory infections are the most common cause of clinical olfactory dysfunction, but the pathogenesis of dysosmia after viral infection is poorly understood. Biopsies of the olfactory mucosa in patients that complain of dysosmia after viral infection fall into two categories: one in which no olfactory epithelium is seen and another in which the epithelium is disordered and populated mainly by immature neurons. We have used intranasal inoculation with an olfactory bulb line variant of MHV to study the consequences of viral infection on peripheral olfactory structures. MHV OBLV has little direct effect on the olfactory epithelium, but causes extensive spongiotic degeneration and destruction of mitral cells and interneurons in the olfactory bulb such that the axonal projection from the bulb via the lateral olfactory tract is markedly reduced. Moreover, surviving mitral cells apparently remain disconnected from the sensory neuron input to the glomerular layer, judging from retrograde labeling studies using Dil. The damage to the bulb indirectly causes a persistent, long-term increase in the turnover of sensory neurons in the epithelium, i.e. the relative proportion of immature to mature sensory neurons and the rate of basal cell proliferation both increase. The changes that develop after inoculation with MHV OBLV closely resemble the disordering of the olfactory epithelium in some patient biopsies. Thus, damage to the olfactory nerve or bulb may contribute to a form of post-viral olfactory dysfunction and MHV OBLV is a useful model for studying the pathogenesis of this form of dysosmia.
Figures


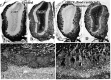




Similar articles
-
Functional consequences following infection of the olfactory system by intranasal infusion of the olfactory bulb line variant (OBLV) of mouse hepatitis strain JHM.Chem Senses. 2001 Oct;26(8):953-63. doi: 10.1093/chemse/26.8.953. Chem Senses. 2001. PMID: 11595672 Free PMC article.
-
Evidence of SARS-CoV2 Entry Protein ACE2 in the Human Nose and Olfactory Bulb.Cells Tissues Organs. 2020;209(4-6):155-164. doi: 10.1159/000513040. Epub 2021 Jan 22. Cells Tissues Organs. 2020. PMID: 33486479 Free PMC article.
-
Distribution of vesicular stomatitis virus proteins in the brains of BALB/c mice following intranasal inoculation: an immunohistochemical analysis.Brain Res. 1994 Jan 28;635(1-2):81-95. doi: 10.1016/0006-8993(94)91426-5. Brain Res. 1994. PMID: 8173982
-
The Olfactory Bulb: An Immunosensory Effector Organ during Neurotropic Viral Infections.ACS Chem Neurosci. 2016 Apr 20;7(4):464-9. doi: 10.1021/acschemneuro.6b00043. Epub 2016 Apr 8. ACS Chem Neurosci. 2016. PMID: 27058872 Free PMC article. Review.
-
Subpopulations of Projection Neurons in the Olfactory Bulb.Front Neural Circuits. 2020 Aug 28;14:561822. doi: 10.3389/fncir.2020.561822. eCollection 2020. Front Neural Circuits. 2020. PMID: 32982699 Free PMC article. Review.
Cited by
-
Smell and taste disorders.Facial Plast Surg Clin North Am. 2004 Nov;12(4):459-68, vii. doi: 10.1016/j.fsc.2004.04.006. Facial Plast Surg Clin North Am. 2004. PMID: 15337114 Free PMC article. Review.
-
Peripheral dendritic cells are essential for both the innate and adaptive antiviral immune responses in the central nervous system.Virology. 2009 Apr 25;387(1):117-26. doi: 10.1016/j.virol.2009.01.032. Epub 2009 Mar 4. Virology. 2009. PMID: 19264338 Free PMC article.
-
Olfactory immune response to SARS-CoV-2.Cell Mol Immunol. 2024 Feb;21(2):134-143. doi: 10.1038/s41423-023-01119-5. Epub 2023 Dec 25. Cell Mol Immunol. 2024. PMID: 38143247 Free PMC article. Review.
-
Microglia are required for protection against lethal coronavirus encephalitis in mice.J Clin Invest. 2018 Mar 1;128(3):931-943. doi: 10.1172/JCI97229. Epub 2018 Jan 29. J Clin Invest. 2018. PMID: 29376888 Free PMC article.
-
Diseases of the nasal cavity.Handb Clin Neurol. 2019;164:285-302. doi: 10.1016/B978-0-444-63855-7.00018-6. Handb Clin Neurol. 2019. PMID: 31604553 Free PMC article. Review.
References
-
- Akerlund, A., Bende, M. and Murphy, C. (1995) Olfactory threshold and nasal mucosal changes in experimentally induced common cold Acta Otolaryngol. (Stockh.), 115,88 -92. - PubMed
-
- Barnett, E.M. and Perlman, S. (1993) The olfactory nerve and not the trigeminal nerve is the major site of CNS entry for mouse hepatitis virus, strain JHM Virology,194 , 185-191. - PubMed
-
- Carr, V.M. and Farbman, A.I. (1992) Ablation of the olfactory bulb up-regulates the rate of neurogenesis and induces precocious cell death in olfactory epithelium Exp. Neurol., 115,55 -59. - PubMed

