Plastid division is driven by a complex mechanism that involves differential transition of the bacterial and eukaryotic division rings
- PMID: 11595800
- PMCID: PMC139157
- DOI: 10.1105/tpc.010185
Plastid division is driven by a complex mechanism that involves differential transition of the bacterial and eukaryotic division rings
Abstract
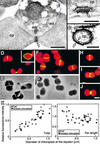
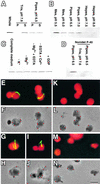
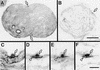
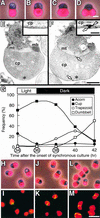
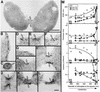
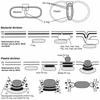
During plastid division, two structures have been detected at the division site in separate analyses. The plastid-dividing ring can be detected by transmission electron microscopy as two (or three) electron-dense rings: an outer ring on the cytosolic face of the outer envelope, occasionally a middle ring in the intermembrane space, and an inner ring on the stromal face of the inner envelope. The FtsZ ring, which plays a central role in bacterial division, also is involved in plastid division and is believed to have descended to plastids from cyanobacterial endosymbiosis. The relationship between the two structures is not known, although there is discussion regarding whether they are identical. Biochemical and immunocytochemical investigations, using synchronized chloroplasts of the red alga Cyanidioschyzon merolae, showed that the plastid FtsZ ring is distinct and separable from the plastid-dividing ring. The FtsZ ring localizes in stroma and faces the inner plastid-dividing ring at the far side from the inner envelope. The FtsZ ring and the inner and outer plastid-dividing rings form in that order before plastid division. The FtsZ ring disappears at the late stage of constriction before dissociation of the plastid-dividing ring, when the constriction is still in progress. Our results suggest that the FtsZ ring;-based system, which originated from a plastid ancestor, cyanobacteria, and the plastid-dividing ring;-based system, which probably originated from host eukaryotic cells, form a complex and are involved in plastid division by distinct modes.
Figures
Similar articles
-
Novel filaments 5 nm in diameter constitute the cytosolic ring of the plastid division apparatus.Plant Cell. 2001 Mar;13(3):707-21. doi: 10.1105/tpc.13.3.707. Plant Cell. 2001. PMID: 11251107 Free PMC article.
-
The discovery of the division apparatus of plastids and mitochondria.J Electron Microsc (Tokyo). 2000;49(1):123-34. doi: 10.1093/oxfordjournals.jmicro.a023776. J Electron Microsc (Tokyo). 2000. PMID: 10791428 Review.
-
A plant-specific dynamin-related protein forms a ring at the chloroplast division site.Plant Cell. 2003 Mar;15(3):655-65. doi: 10.1105/tpc.009373. Plant Cell. 2003. PMID: 12615939 Free PMC article.
-
The timing and manner of disassembly of the apparatuses for chloroplast and mitochondrial division in the red alga Cyanidioschyzon merolae.Planta. 2001 Mar;212(4):517-28. doi: 10.1007/s004250000426. Planta. 2001. PMID: 11525508
-
Structure, function and evolution of the mitochondrial division apparatus.Biochim Biophys Acta. 2006 May-Jun;1763(5-6):510-21. doi: 10.1016/j.bbamcr.2006.03.007. Epub 2006 Apr 5. Biochim Biophys Acta. 2006. PMID: 16690143 Review.
Cited by
-
ARC5, a cytosolic dynamin-like protein from plants, is part of the chloroplast division machinery.Proc Natl Acad Sci U S A. 2003 Apr 1;100(7):4328-33. doi: 10.1073/pnas.0530206100. Epub 2003 Mar 17. Proc Natl Acad Sci U S A. 2003. PMID: 12642673 Free PMC article.
-
An Arabidopsis homolog of the bacterial cell division inhibitor SulA is involved in plastid division.Plant Cell. 2004 Jul;16(7):1801-11. doi: 10.1105/tpc.022335. Epub 2004 Jun 18. Plant Cell. 2004. PMID: 15208387 Free PMC article.
-
The plastid division proteins, FtsZ1 and FtsZ2, differ in their biochemical properties and sub-plastidial localization.Biochem J. 2005 May 1;387(Pt 3):669-76. doi: 10.1042/BJ20041281. Biochem J. 2005. PMID: 15601251 Free PMC article.
-
Prospective function of FtsZ proteins in the secondary plastid of chlorarachniophyte algae.BMC Plant Biol. 2015 Nov 10;15:276. doi: 10.1186/s12870-015-0662-7. BMC Plant Biol. 2015. PMID: 26556725 Free PMC article.
-
Paths toward algal genomics.Plant Physiol. 2005 Feb;137(2):410-27. doi: 10.1104/pp.104.053447. Plant Physiol. 2005. PMID: 15710682 Free PMC article. Review. No abstract available.
References
-
- Beech, P.L., and Gilson, P.R. (2000). FtsZ and organelle division in protists. Protist 151, 11–16. - PubMed
-
- Beech, P.L., Nheu, T., Schultz, T., Herbert, S., Lithgow, T., Gilson, P.R., and McFadden, G.I. (2000). Mitochondrial FtsZ in a chromophyte alga. Science 287, 1276–1279. - PubMed
-
- Bramhill, D. (1997). Bacterial cell division. Annu. Rev. Cell Dev. Biol. 13, 395–424. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

