Identification and characterization of GONST1, a golgi-localized GDP-mannose transporter in Arabidopsis
- PMID: 11595802
- PMCID: PMC139159
- DOI: 10.1105/tpc.010247
Identification and characterization of GONST1, a golgi-localized GDP-mannose transporter in Arabidopsis
Abstract
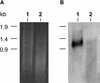
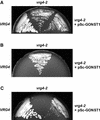
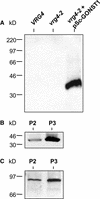
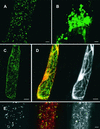
Transport of nucleotide sugars across the Golgi apparatus membrane is required for the luminal synthesis of a variety of plant cell surface components. We identified an Arabidopsis gene encoding a nucleotide sugar transporter (designated GONST1) that we have shown by transient gene expression to be localized to the Golgi. GONST1 complemented a GDP-mannose transport-defective yeast mutant (vrg4-2), and Golgi-rich vesicles from the complemented strain displayed increased GDP-mannose transport activity. GONST1 promoter::beta-glucuronidase studies suggested that this gene is expressed ubiquitously. The identification of a Golgi-localized nucleotide sugar transporter from plants will allow the study of the importance of this class of proteins in the synthesis of plant cell surface components such as cell wall polysaccharides.
Figures




References
-
- Abeijon, C., Mandon, E.C., and Hirschberg, C.B. (1997). Transporters of nucleotide sugars, nucleotide sulfate and ATP in the Golgi apparatus. Trends Biochem. Sci. 22, 203–207. - PubMed
-
- Agatep, R., Kirkpatrick, R.D., Parchaliuk, D.L., Woods, R.A., and Gietz, R.D. (1998). Transformation of Saccharomyces cerevisiae by the lithium acetate/single-stranded carrier DNA/polyethylene glycol protocol. Technical Tips Online (http://tto.trends.com). September 14, 2001.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

