A novel membrane protein that is transported to protein storage vacuoles via precursor-accumulating vesicles
- PMID: 11595807
- PMCID: PMC139164
- DOI: 10.1105/tpc.010171
A novel membrane protein that is transported to protein storage vacuoles via precursor-accumulating vesicles
Abstract
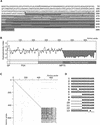
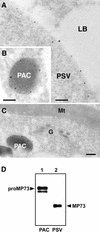
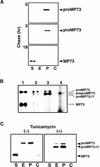
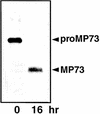
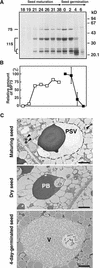
A novel protein, MP73, was specifically found on the membrane of protein storage vacuoles of pumpkin seed. MP73 appeared during seed maturation and disappeared rapidly after seed germination, in association with the morphological changes of the protein storage vacuoles. The MP73 precursor deduced from the isolated cDNA was composed of a signal peptide, a 24-kD domain (P24), and the MP73 domain with a putative long alpha-helix of 13 repeats that are rich in glutamic acid and arginine residues. Immunocytochemistry and immunoblot analysis showed that the precursor-accumulating (PAC) vesicles (endoplasmic reticulum-derived vesicles responsible for the transport of storage proteins) accumulated proMP73, but not MP73, on the membranes. Subcellular fractionation of the pulse-labeled maturing seed demonstrated that the proMP73 form with N-linked oligosaccharides was synthesized on the endoplasmic reticulum and then transported to the protein storage vacuoles via PAC vesicles. Tunicamycin treatment of the seed resulted in the efficient deposition of proMP73 lacking the oligosaccharides (proMP73 Delta Psi) into the PAC vesicles but no accumulation of MP73 in vacuoles. Tunicamycin might impede the transport of proMP73 Delta Psi from the PAC vesicles to the vacuoles or might make the unglycosylated protein unstable in the vacuoles. After arrival at protein storage vacuoles, proMP73 was cleaved by the action of a vacuolar enzyme to form a 100-kD complex on the vacuolar membranes. These results suggest that PAC vesicles might mediate the delivery of not only storage proteins but also membrane proteins of the vacuoles.
Figures



Similar articles
-
A vacuolar sorting receptor PV72 on the membrane of vesicles that accumulate precursors of seed storage proteins (PAC vesicles).Plant Cell Physiol. 2002 Oct;43(10):1086-95. doi: 10.1093/pcp/pcf152. Plant Cell Physiol. 2002. PMID: 12407187
-
A pumpkin 72-kDa membrane protein of precursor-accumulating vesicles has characteristics of a vacuolar sorting receptor.Plant Cell Physiol. 1997 Dec;38(12):1414-20. doi: 10.1093/oxfordjournals.pcp.a029138. Plant Cell Physiol. 1997. PMID: 9522472
-
Vesicle transport and processing of the precursor to 2S albumin in pumpkin.Plant J. 1993 Nov;4(5):793-800. doi: 10.1046/j.1365-313x.1993.04050793.x. Plant J. 1993. PMID: 8275099
-
Plant Vacuoles.Annu Rev Plant Biol. 2018 Apr 29;69:123-145. doi: 10.1146/annurev-arplant-042817-040508. Epub 2018 Mar 21. Annu Rev Plant Biol. 2018. PMID: 29561663 Review.
-
The riddle of the plant vacuolar sorting receptors.Protoplasma. 2005 Dec;226(3-4):103-8. doi: 10.1007/s00709-005-0117-3. Epub 2005 Dec 12. Protoplasma. 2005. PMID: 16333569 Review.
Cited by
-
Vacuolar targeting of recombinant antibodies in Nicotiana benthamiana.Plant Biotechnol J. 2016 Dec;14(12):2265-2275. doi: 10.1111/pbi.12580. Epub 2016 Jun 14. Plant Biotechnol J. 2016. PMID: 27159528 Free PMC article.
-
MAIGO2 is involved in exit of seed storage proteins from the endoplasmic reticulum in Arabidopsis thaliana.Plant Cell. 2006 Dec;18(12):3535-47. doi: 10.1105/tpc.106.046151. Epub 2006 Dec 28. Plant Cell. 2006. PMID: 17194767 Free PMC article.
-
Characterization of vacuolar transport of the endogenous alkaloid berberine in Coptis japonica.Plant Physiol. 2005 Aug;138(4):1939-46. doi: 10.1104/pp.105.064352. Epub 2005 Jul 15. Plant Physiol. 2005. PMID: 16024684 Free PMC article.
-
Phosphorylation of the 12 S globulin cruciferin in wild-type and abi1-1 mutant Arabidopsis thaliana (thale cress) seeds.Biochem J. 2007 Jun 1;404(2):247-56. doi: 10.1042/BJ20061569. Biochem J. 2007. PMID: 17313365 Free PMC article.
-
Selective membrane protein internalization accompanies movement from the endoplasmic reticulum to the protein storage vacuole pathway in Arabidopsis.Plant Cell. 2005 Nov;17(11):3066-80. doi: 10.1105/tpc.105.035212. Epub 2005 Oct 14. Plant Cell. 2005. PMID: 16227454 Free PMC article.
References
-
- Chou, P.Y., and Fasman, G.D. (1974). Prediction of protein conformation. Biochemistry 13, 222–245. - PubMed
-
- Craig, S., and Staehelin, L.A. (1998). High pressure freezing of intact plant tissues: Evaluation and characterization of novel features of the endoplasmic reticulum and associated membrane systems. Eur. J. Cell Biol. 46, 80–93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

