A sperm ion channel required for sperm motility and male fertility
- PMID: 11595941
- PMCID: PMC8462998
- DOI: 10.1038/35098027
A sperm ion channel required for sperm motility and male fertility
Abstract
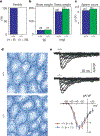
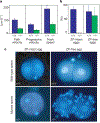
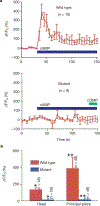
Calcium and cyclic nucleotides have crucial roles in mammalian fertilization, but the molecules comprising the Ca2+-permeation pathway in sperm motility are poorly understood. Here we describe a putative sperm cation channel, CatSper, whose amino-acid sequence most closely resembles a single, six-transmembrane-spanning repeat of the voltage-dependent Ca2+-channel four-repeat structure. CatSper is located specifically in the principal piece of the sperm tail. Targeted disruption of the gene results in male sterility in otherwise normal mice. Sperm motility is decreased markedly in CatSper-/- mice, and CatSper-/- sperm are unable to fertilize intact eggs. In addition, the cyclic-AMP-induced Ca2+ influx is abolished in the sperm of mutant mice. CatSper is thus vital to cAMP-mediated Ca2+ influx in sperm, sperm motility and fertilization. CatSper represents an excellent target for non-hormonal contraceptives for both men and women.
Figures





Comment in
-
Ion channels. Swimming with sperm.Nature. 2001 Oct 11;413(6856):579, 581-2. doi: 10.1038/35098164. Nature. 2001. PMID: 11595929 No abstract available.
References
-
- Wassarman PM, Jovine L& Litscheer ES Aprofile of fertilization inmammals. Nature Cell Biol. 3, E59–E64 (2001). - PubMed
-
- Yanagimachi R in The Physiology of Reproduction (eds Knobill E & Neill JD ) 189–315 (Raven, New York, 1994).
-
- Jungnickel MK,Marrero H, Birnbaumer L, Lemos JR & Florman HM Trp2 regulates entry of Ca2+ into mouse sperm triggered by egg ZP3. Nature Cell Biol. 3, 499–502 (2001). - PubMed
-
- Bedford JM Mammalian fertilization misread? Sperm penetration of the eutherian zona pellucida is unlikely to be a lytic event. Biol. Reprod. 59, 1275–1287 (1998). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

